A w/o/w adjuvant composition, prepared vaccine composition and preparation method thereof
A vaccine composition and composition technology, applied in the direction of biochemical equipment and methods, vaccines, drug combinations, etc., can solve the problems of easy inactivation of antigens, poor stability, high viscosity, etc., and achieve the effect of ensuring the immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0038] As an embodiment of the present invention, the w / o / w adjuvant composition of the above composition further comprises water.
[0039] As an embodiment of the present invention, in the w / o / w adjuvant composition of the present invention, the w / o / w adjuvant composition further includes 0.5%W / W-3% by weight w / w water.
[0040] 1.0% W / W, 1,5% W / W, 2.0% W / W water may also be included in the w / o / w adjuvant composition of the present invention.
[0041] After the above-mentioned content of water is added to the w / o / w adjuvant composition of the present invention, the cold stability of the adjuvant composition is greatly improved.
[0042] As an embodiment of the present invention, in the w / o / w adjuvant composition of the present invention, the oil includes mineral oil, vegetable oil or synthetic metabolizable oil.
[0043] As a preferred embodiment of the present invention, in the w / o / w adjuvant composition of the present invention, the mineral oil is white oil for veterinary...
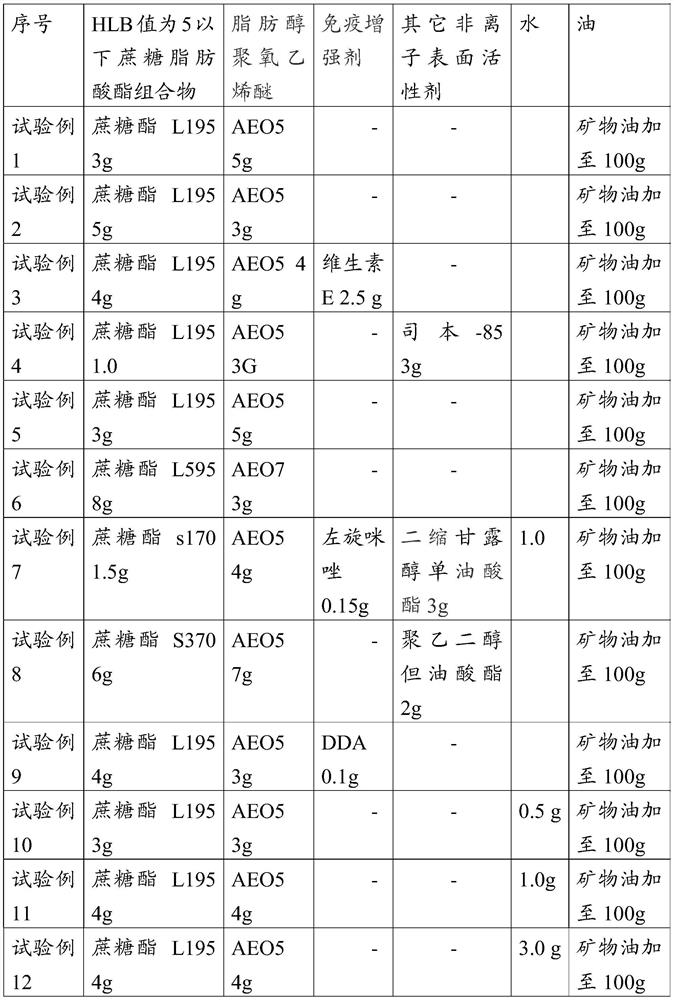
Embodiment 1
[0071] Embodiment 1, the preparation of adjuvant composition
[0072] Sucrose ester L195 (Mitsubishi Ryoto) 4.0g, AEO-5 4.0g, mineral oil 92g
[0073] Preparation method: Mix sucrose ester L195, AEO-5 and mineral oil evenly, and heat to 30°C.
Embodiment 2
[0074] Embodiment 2, vaccine composition
[0075] Circovirus antigen solution 50g (virus content before inactivation is 10 7.2 TCID 50 ), 50 g of the adjuvant composition prepared in Example 1 were first heated to 31° C., and then in a water bath, the stirring was started at a speed of 350 rpm, and the circovirus antigen solution was added to the adjuvant composition, stirred and emulsified The time is 10 minutes, and the stirring is completed. Put to 20 ℃. Particle size determination and stability tests were then carried out. The particle size was measured using a Malvern 3000 laser particle size analyzer, and IKAR W20 was used for stirring.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com