Streptococcus suis △cps/ssna-msly(p353l)-sc19 engineering bacteria and its application in vaccines
A-SC19, Streptococcus suis technology, applied in the field of veterinary biological products, can solve the problems of high cost, low immune efficacy, strong virulence, etc., and achieve the effects of reducing side effects, improving safety, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
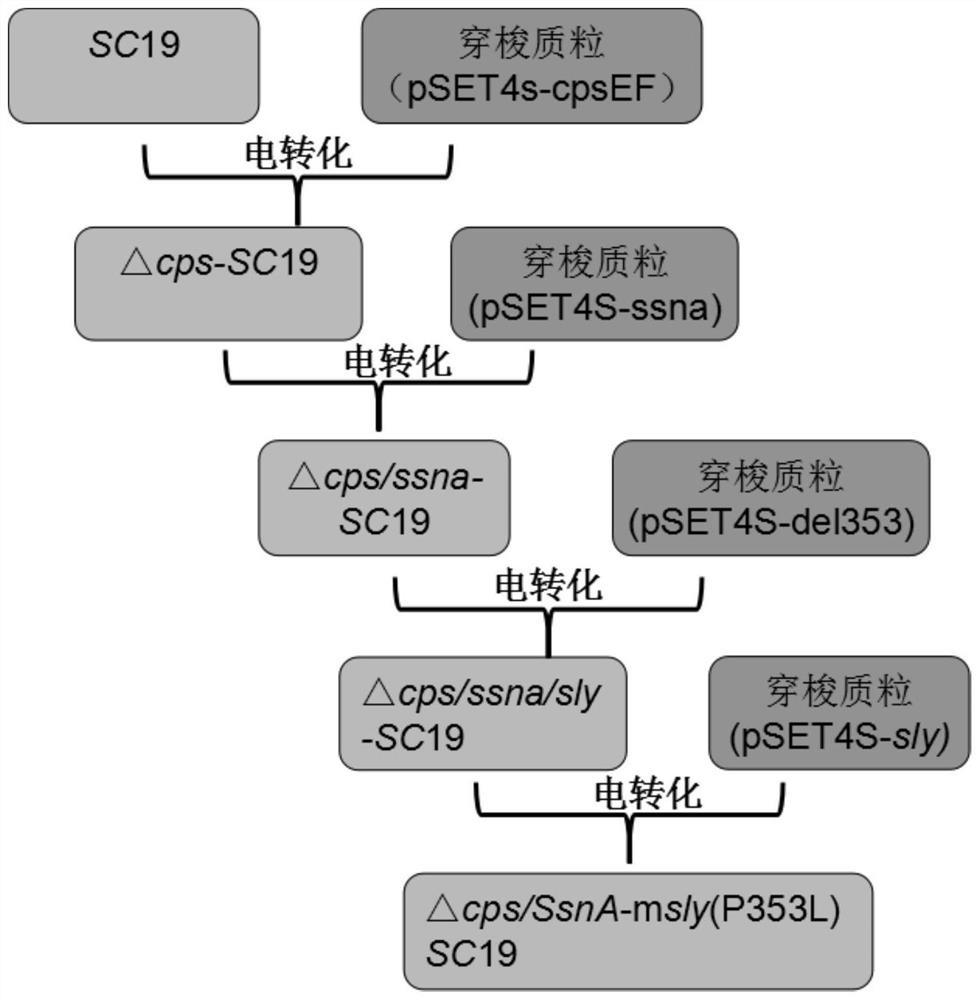
[0101] 1. △CPS / SsnA-mSly(P353L)-SC19 engineering bacteria
[0102] Specific construction strategies such as figure 1 As shown, the construction method includes the following steps:
[0103] 1) According to the ORF sequence of the cpsEF gene in the whole genome sequence of Streptococcus suis type 2 05ZYH33 strain (GenBank: CP000407.1) published on NCBI such as SEQ ID No.1, the ORF sequence of the ssna gene is shown as SEQ ID No. 2 and the ORF sequence of the sly gene, such as SEQ ID No.3, design primers for knocking out the cpsEF gene and the ssna gene, and construct a primer pair for the point mutation of the sly gene, respectively:
[0104] a. Construction of primers for knocking out the cpsEF gene:
[0105] Upstream homology arm forward and reverse primers:
[0106] ECORI-E-1: AAAGAATTCGGCTCGTGCTATATTCTCTTGG,
[0107] RONGHE-EF-2:GAATCTTTTTCATAACAGCTCTCTCCACTATTTC;
[0108] Downstream homology arm forward and reverse primers:
[0109] RONGHE-EF-1:
[0110] GAAATAGTGGA...
Embodiment 2
[0230] Example 2: The safety of the inactivated vaccine prepared with △CPS / SsnA-mSly(P353L)-SC19 and the immune protection effect to multiple serotype Streptococcus suis infection
[0231] 1. Preparation of inactivated vaccine using △CPS / SsnA-mSly(P353L)-SC19 strain as antigen
[0232] (1) Bacterial solution preparation
[0233] Pick △CPS / SsnA-mSly(P353L)-SC19 from the plate and culture it overnight in TSB (containing 5% serum), and then transfer the bacteria cultured to the stationary phase to 300ml TSB (containing 5% serum) at a ratio of 1:100. % serum), cultured for 12 hours.
[0234] (2) Bacterial antigen inactivation
[0235] Bacteria liquid that has been cultivated to the stable period: take out 1ml for bacterial purity detection and counting, and conduct inspection according to the appendix of the current "Chinese Veterinary Pharmacopoeia". The result shows that the number of bacteria in each ml of bacterial liquid is ; the remaining bacteria are inactivated by addin...
Embodiment 3
[0257] Example 3: The safety of the attenuated vaccine prepared with △CPS / SsnA-mSly(P353L)-SC19 and the immune protection effect to multiple serotypes of Streptococcus suis infection
[0258] 1. Safety test of △CPS / SsnA-mSly(P353L)-SC19 as an attenuated vaccine strain
[0259] Pick a single colony of the wild strain SC19 and the mutant strain △CPS / SsnA-mSly(P353L)-SC19 from the fresh plate cultured overnight, place it in 5ml TSB (containing 5% serum) and place it at 37 degrees at 160 rpm for 12 hours, and a blank control was set. During the cultivation process, 100 μl was taken out from each group every hour to measure the light absorption value at OD600, and the growth curve was drawn. The experiment was repeated three times, and it was observed that there was no significant difference in the growth rate between the wild strain and the mutant, indicating that the mutation did not affect the growth characteristics of Streptococcus suis in the medium. Bacterial counts in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com