Swine Mycoplasma Pneumonia Vaccine Strain
A technology of mycoplasma pneumoniae and mycoplasma hyopneumoniae, which is applied in the direction of viruses/bacteriophages, microorganisms, biochemical equipment and methods, etc., can solve the problems of affecting the growth and development of pigs and feed conversion rate, destroying the mucosa-cilia barrier, and lack of effective drugs, etc. , to achieve the effects of ensuring immune efficacy, reducing adverse reactions, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
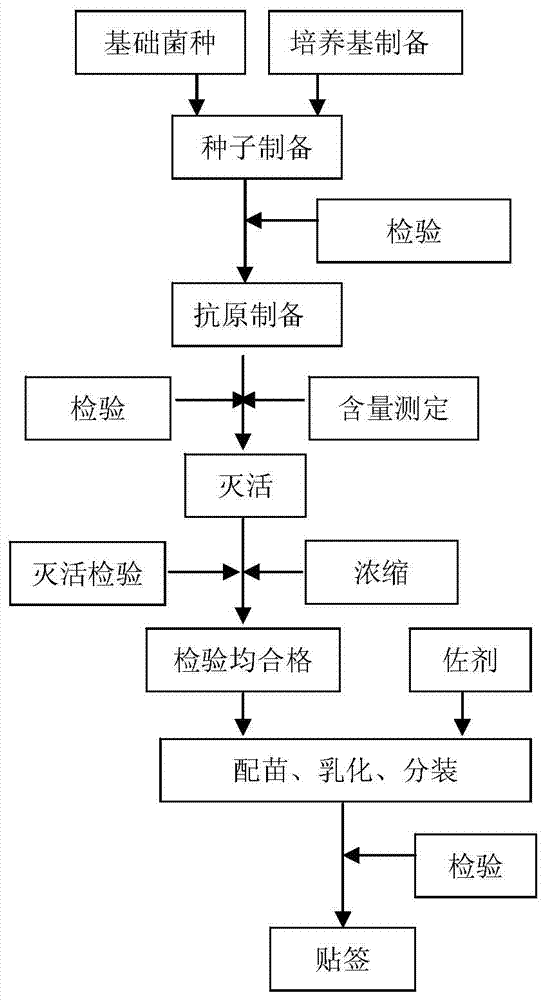
Image
Examples
Embodiment 1
[0017] Embodiment 1: the breeding of Mycoplasma hyopneumoniae NJ strain
[0018] 1 strain isolation and culture
[0019] After the diseased lung tissue was ground, it was added to a liquid medium (pH 7.4-7.6), and cultured at 37°C for 3-10 days, then the pH dropped to 6.6-7.0, and it became slightly uniform and turbid. Then inoculate the solid medium and culture it at 37°C for 3 to 10 days, and fried egg-like colonies can be seen. After passage on the solid medium for 3 times, inoculate the liquid medium to obtain Mycoplasma hyopneumoniae.
[0020] 2 identification
[0021] The isolated Mycoplasma hyopneumoniae strains were cultured at 37°C for 3-10 days, and then the pH value dropped to 6.6-7.0, with slight uniform turbidity. The smear was carried out, and typical Mycoplasma hyopneumoniae cells were seen under the staining microscope. Then inoculate solid culture medium and culture at 37°C for 3-10 days, and fried egg-like colonies can be seen. Consistent with the culture ...
Embodiment 2
[0029] Example 2: The preparation method of the inactivated vaccine (NJ strain) of mycoplasma pneumoniae in swine.
[0030] 1 strain culture
[0031] Mycoplasma hyopneumoniae NJ strain was selected as the strain, which was isolated and identified by us and obtained after subculture. This strain was sent to the China Center for Type Culture Collection (CCTCC) on July 16, 2012, with the preservation number: CCTCCM2012286.
[0032] 2 strain characteristics
[0033] 2.1 Morphological and biochemical characteristics This bacterium is a Gram-negative polymorphic microorganism, which has ring, spherical, point, rod and bipolar shapes, is not easy to color, and has no cell wall. The biochemical characteristics should conform to the characteristics of the bacteria in bacterial taxonomy.
[0034] 2.2 Culture characteristics In the liquid culture medium (pH value 7.4-7.6), after cultured at 37°C for 3-7 days, the pH value will drop to 6.6-7.0, slightly uniform and turbid, and the titer...
Embodiment 3
[0059] Example 3: Observation of immune effect.
[0060] 1 vaccine
[0061] Mycoplasma pneumonia inactivated vaccine (NJ strain) oil adjuvant, Mycoplasma pneumonia inactivated vaccine (NJ strain) ISA11RVG, Mycoplasma pneumonia inactivated vaccine (NJ strain) Gel01ST, Mycoplasma pneumonia inactivated vaccine produced by a foreign company.
[0062] 2 strong poison for inspection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com