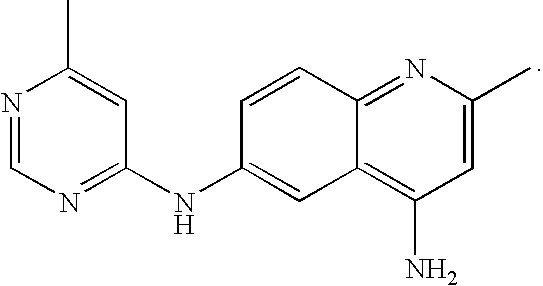
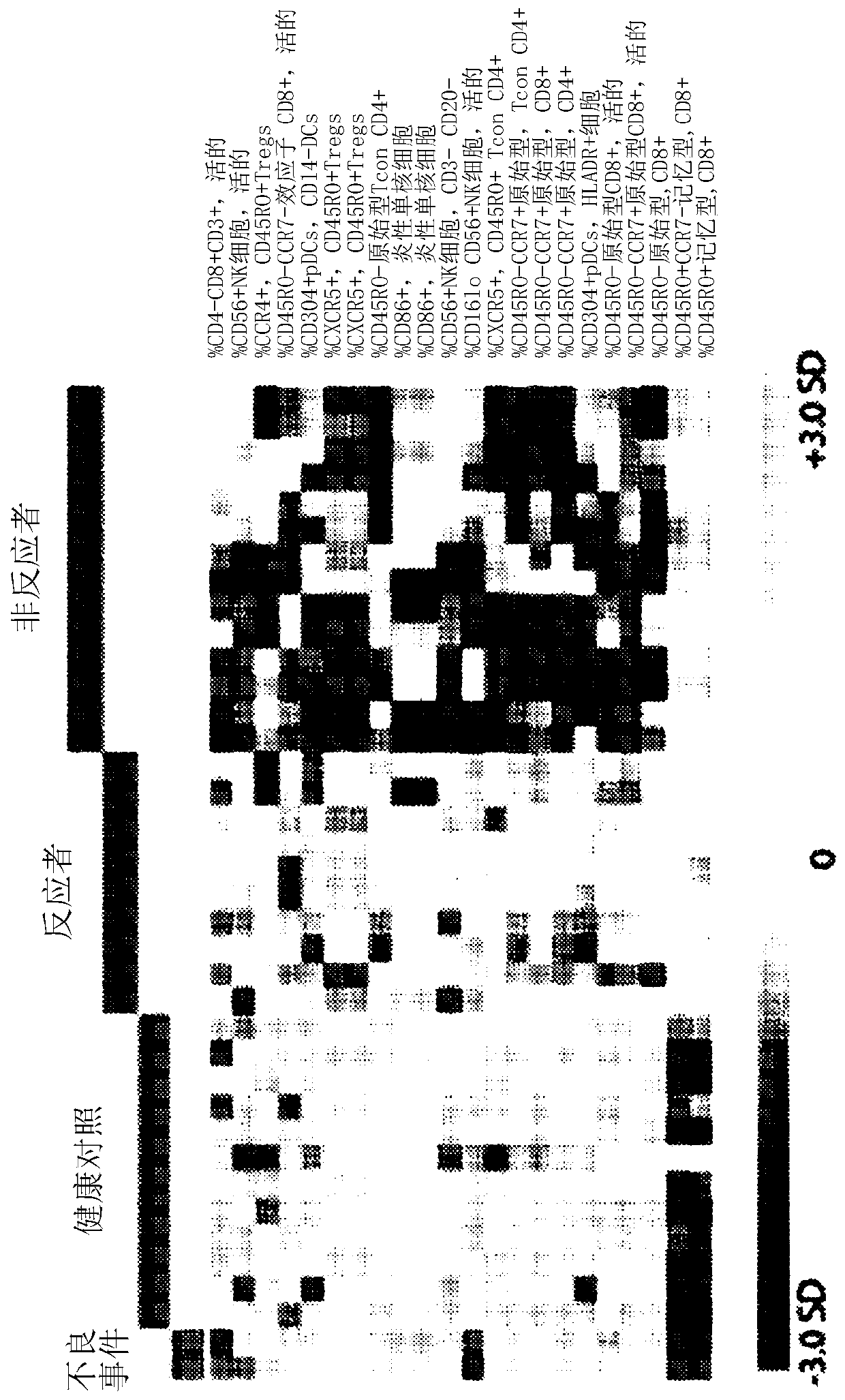
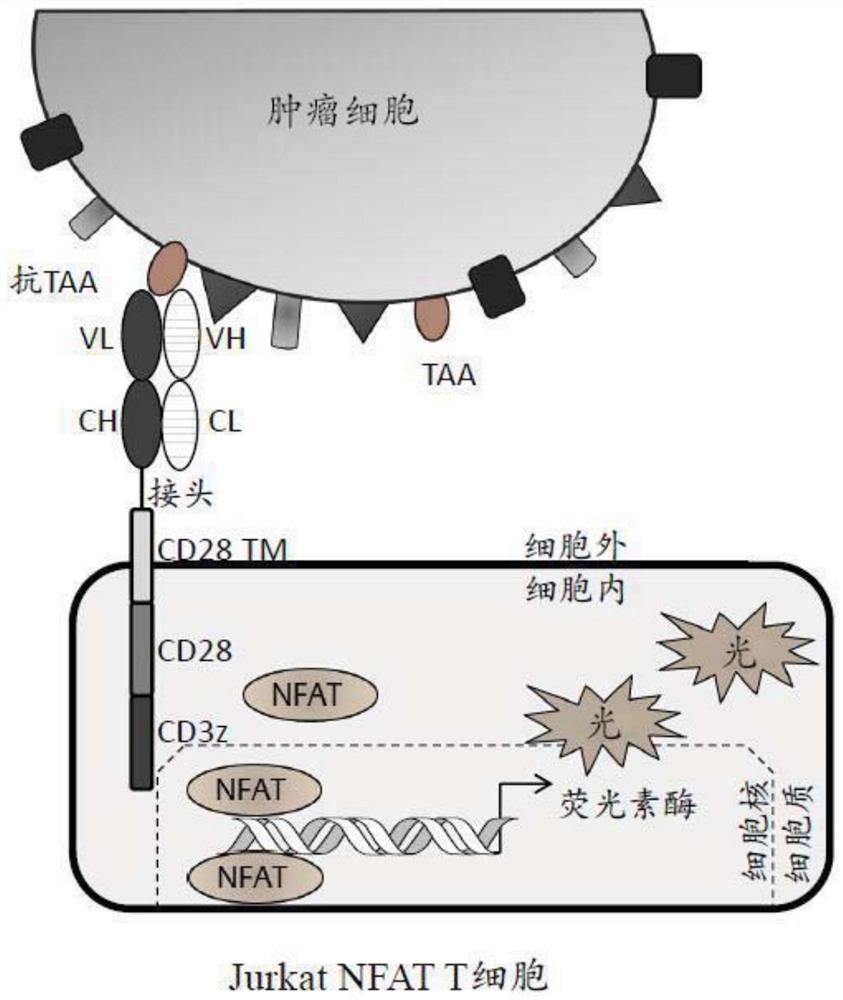
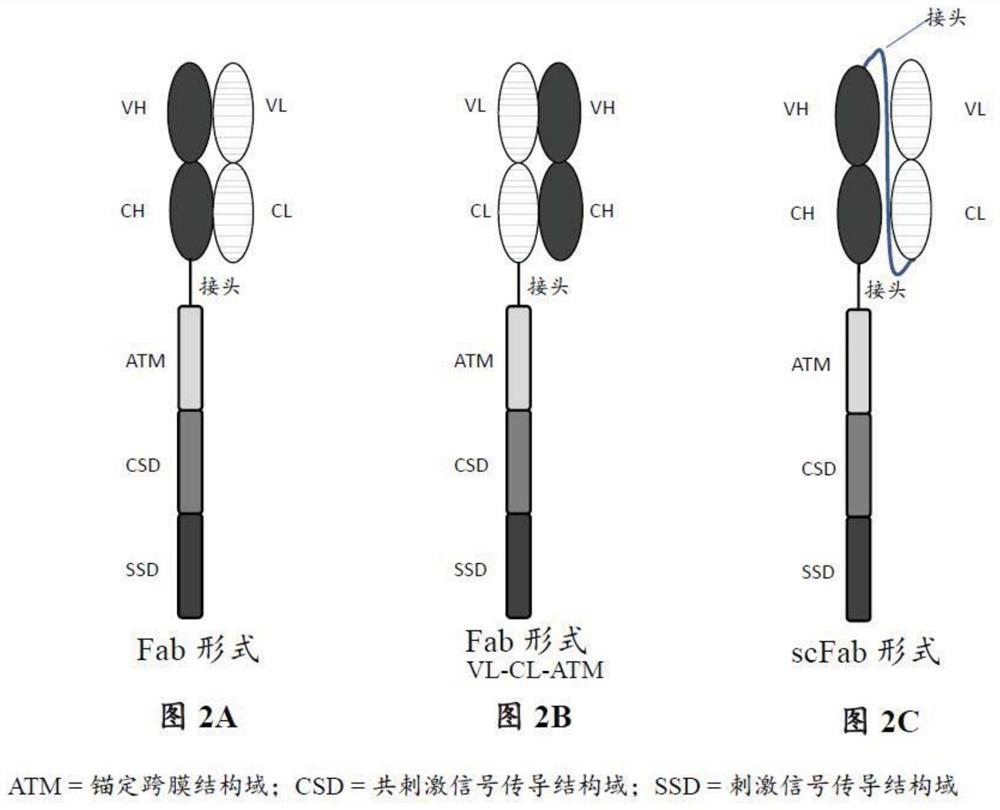
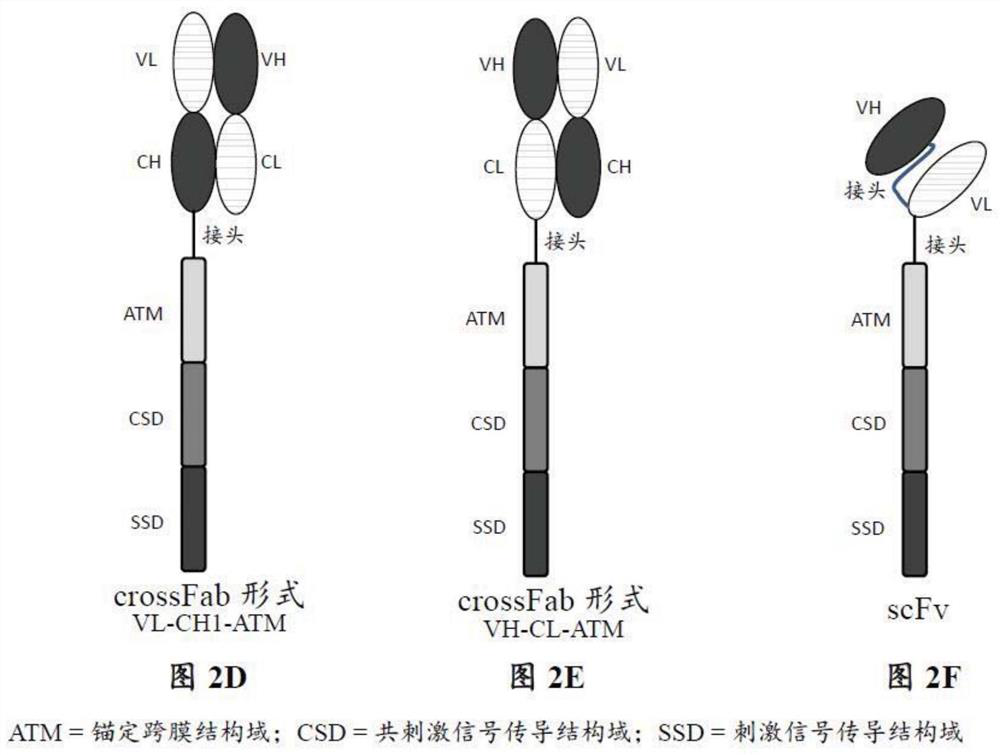
Patents
Literature
36 results about "Clinical reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Predicting patient responsiveness to immune checkpoint inhibitors
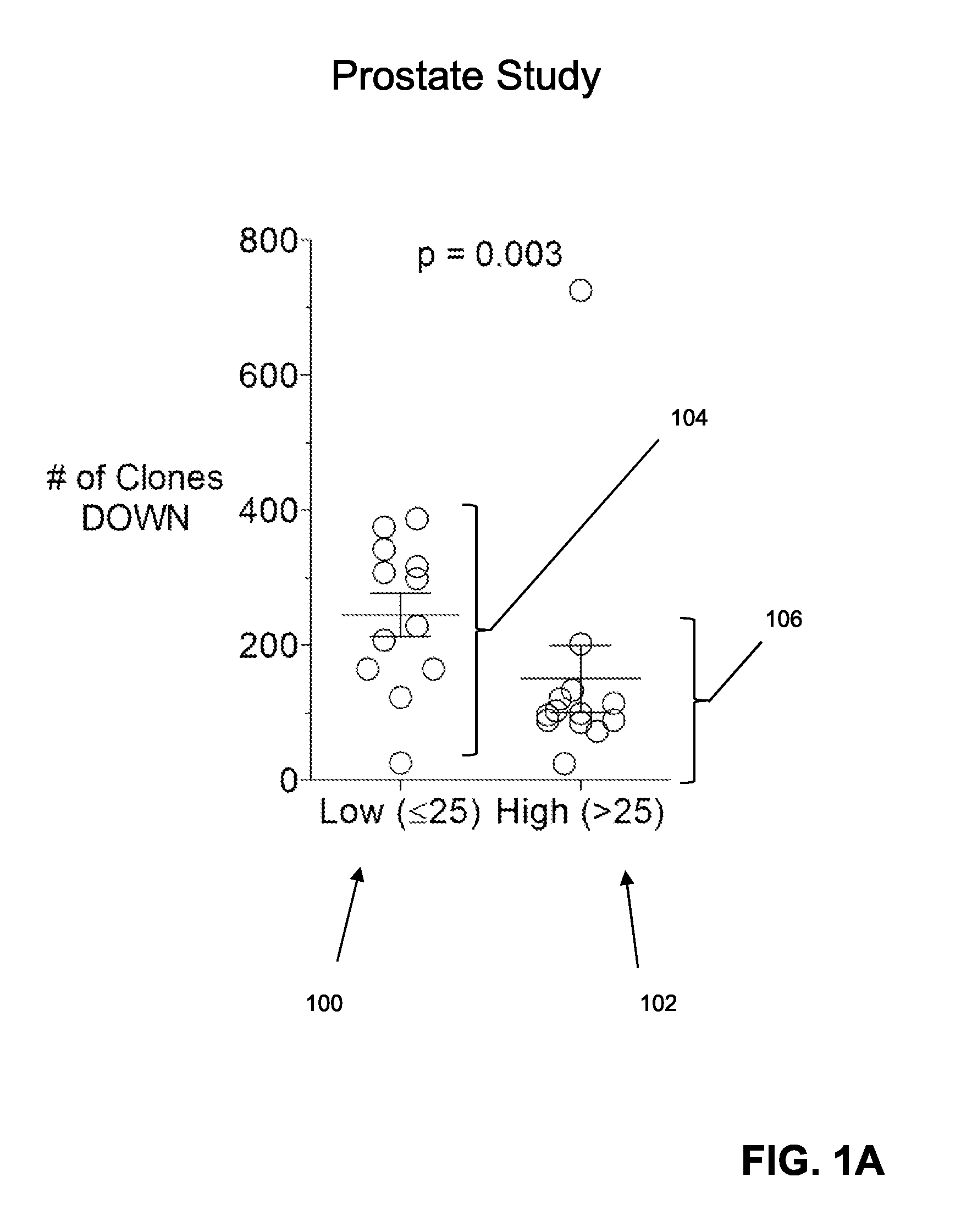
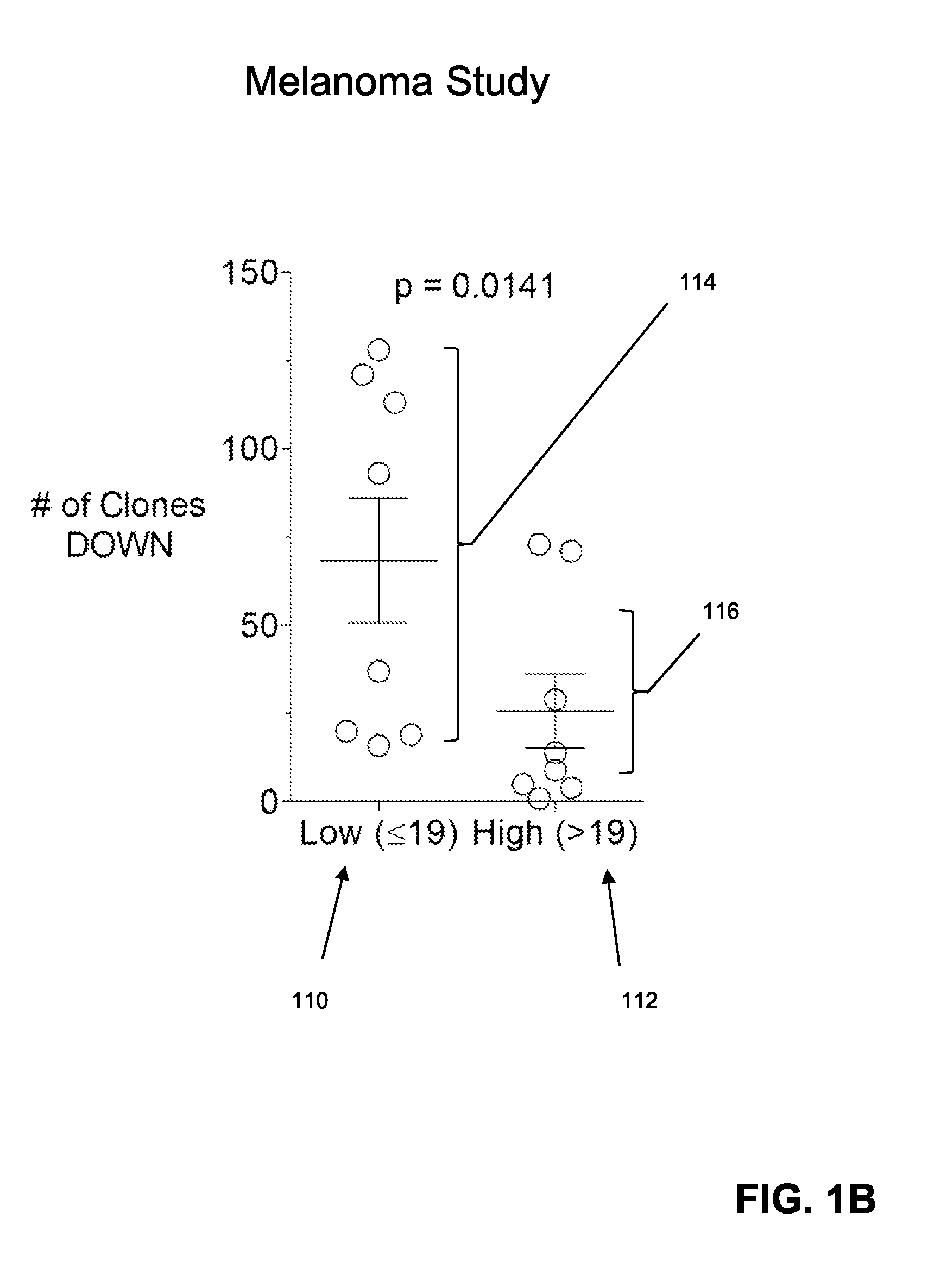
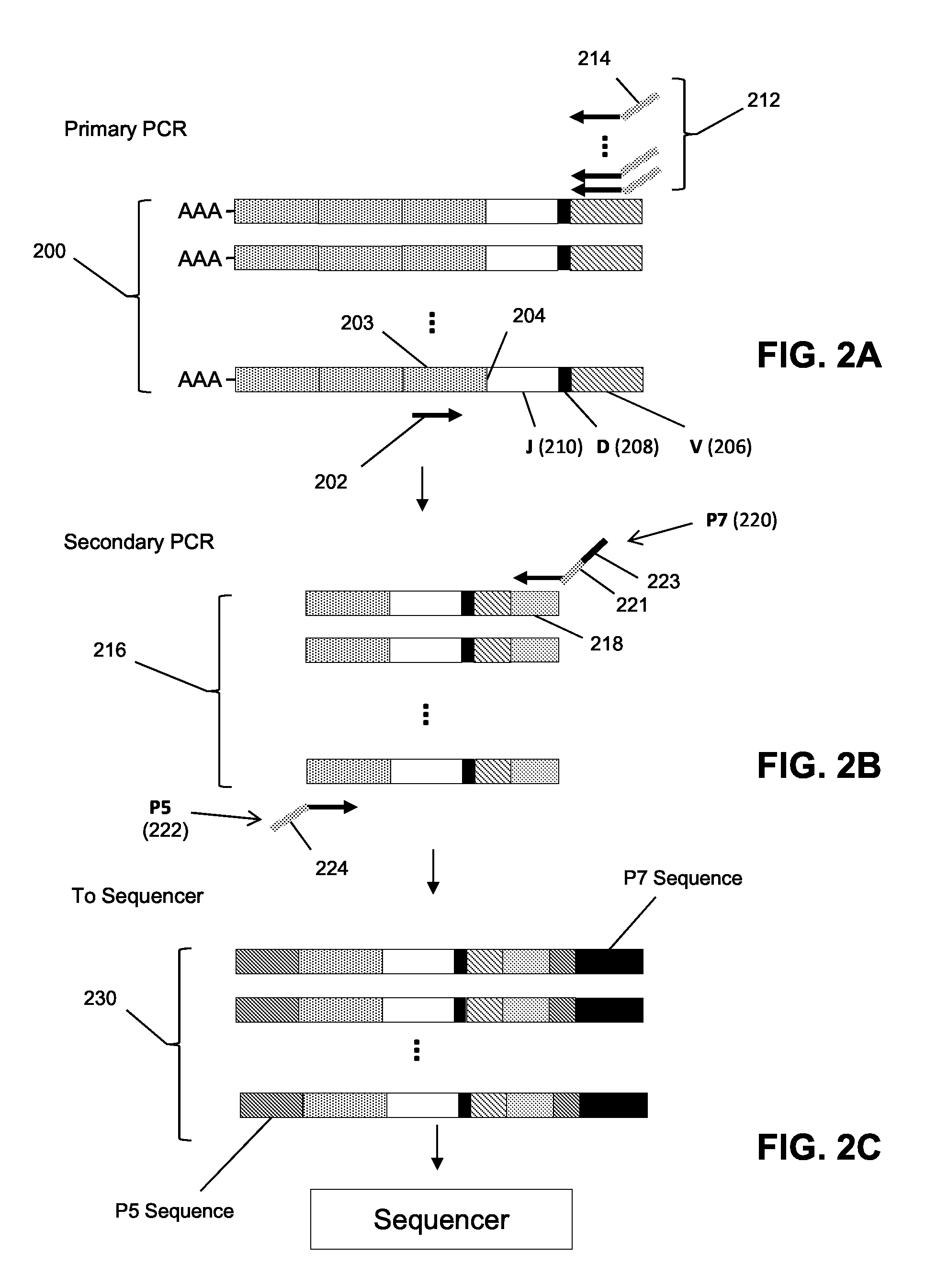
The invention is directed to a method of predicting clinical response of a patient to treatment of a cancer by an immune checkpoint pathway inhibitor, such as an anti-CTLA-4 or anti-PD-1 antibody binding compound. In one aspect the method comprises generating pre- and post-treatment clonotype profiles, determining a number of clonotypes that decrease in frequency between the first and second clonotype profiles, and predicting a lack of responsiveness in the patient to the treatment whenever the number of clonotypes that decrease in frequency is greater than a predetermined value.
Owner:ADAPTIVE BIOTECH +1
Pills for treating rheumatic heart disease and preparation method thereof
The invention discloses pills for treating a rheumatic heart disease and a preparation method thereof and belongs to the field of traditional Chinese medicines. Active ingredients of the pills comprise: by weight, 50 to 70 parts of cassia twig, 50 to 65 parts of mix-fried licorice root, 45 to 60 parts of dwarf lilyturf tuber, 45 to 60 parts of Chinese magnoliavine fruit, 40 to 55 parts of poria cocos, 40 to 50 parts of mix-fried milkvetch root, 40 to 50 parts of curcumae, 35 to 45 parts of pubescent holly root, 35 to 45 parts of red sage root, 30 to 40 parts of Chinese arborvitae kernel, 25 to 40 parts of snakegourd fruit, 20 to 30 parts of longan pulp, 20 to 30 parts of sichuan lovage rhizome, 15 to 30 parts of stir-fried milkwort root, 15 to 25 parts of grassleaf sweetflag rhizome and 10 to 25 parts of syzygium jambos seed. The pills utilizes the unique traditional Chinese medicines, has a good formula, has effects of tonifying qi, nourishing yin, dispelling wind, dissipating heat, relaxing muscles and tendons, invigorating the circulation of blood, strengthening vital qi and eliminating pathogens, and has good absorption effects, obvious curative effects, no toxic and side effect and no adverse clinical reactions. A clinical experiment proves that the pills have good effects of treatment on a rheumatic heart disease, and postoperative recovery.
Owner:刘青
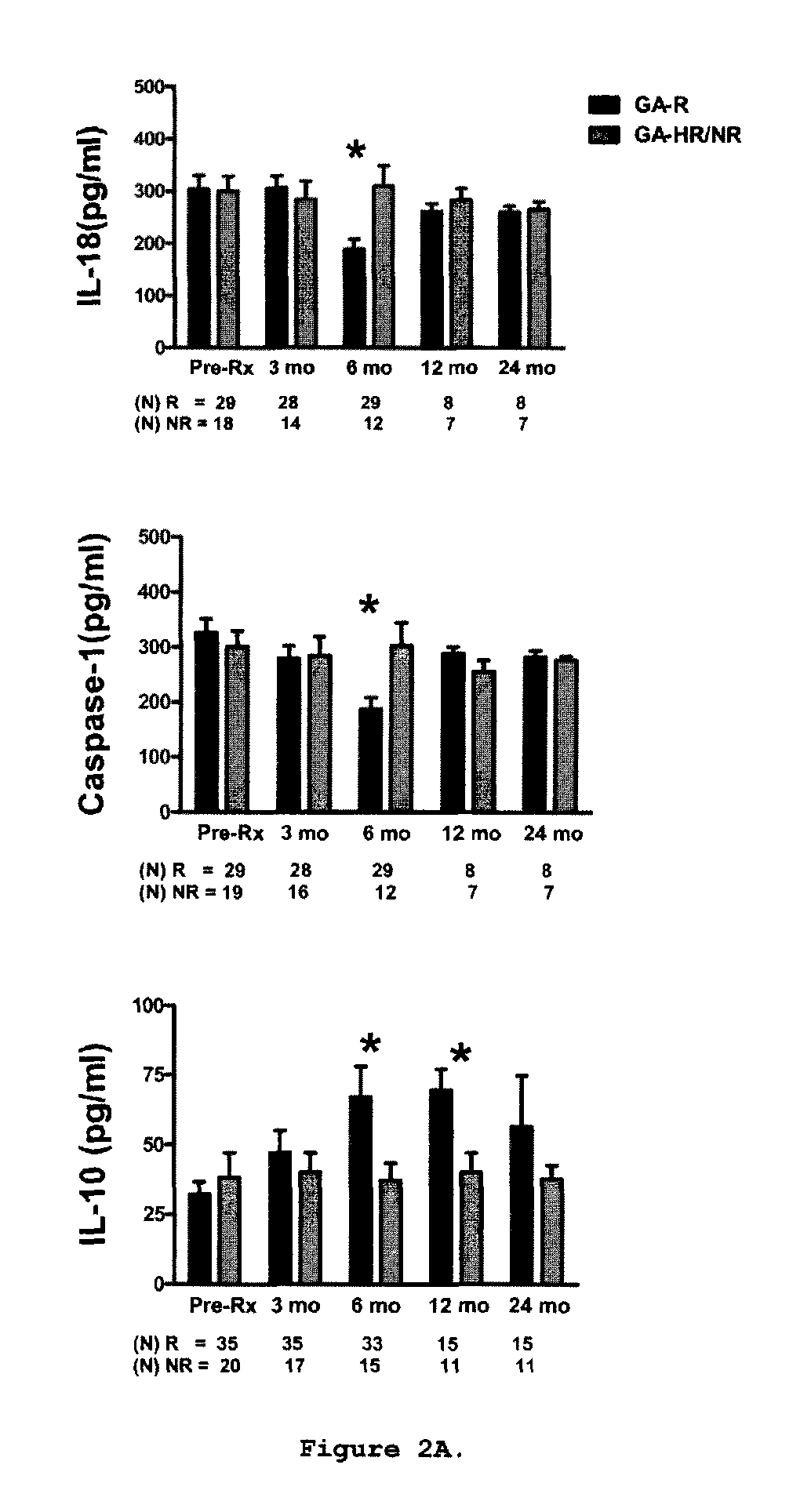
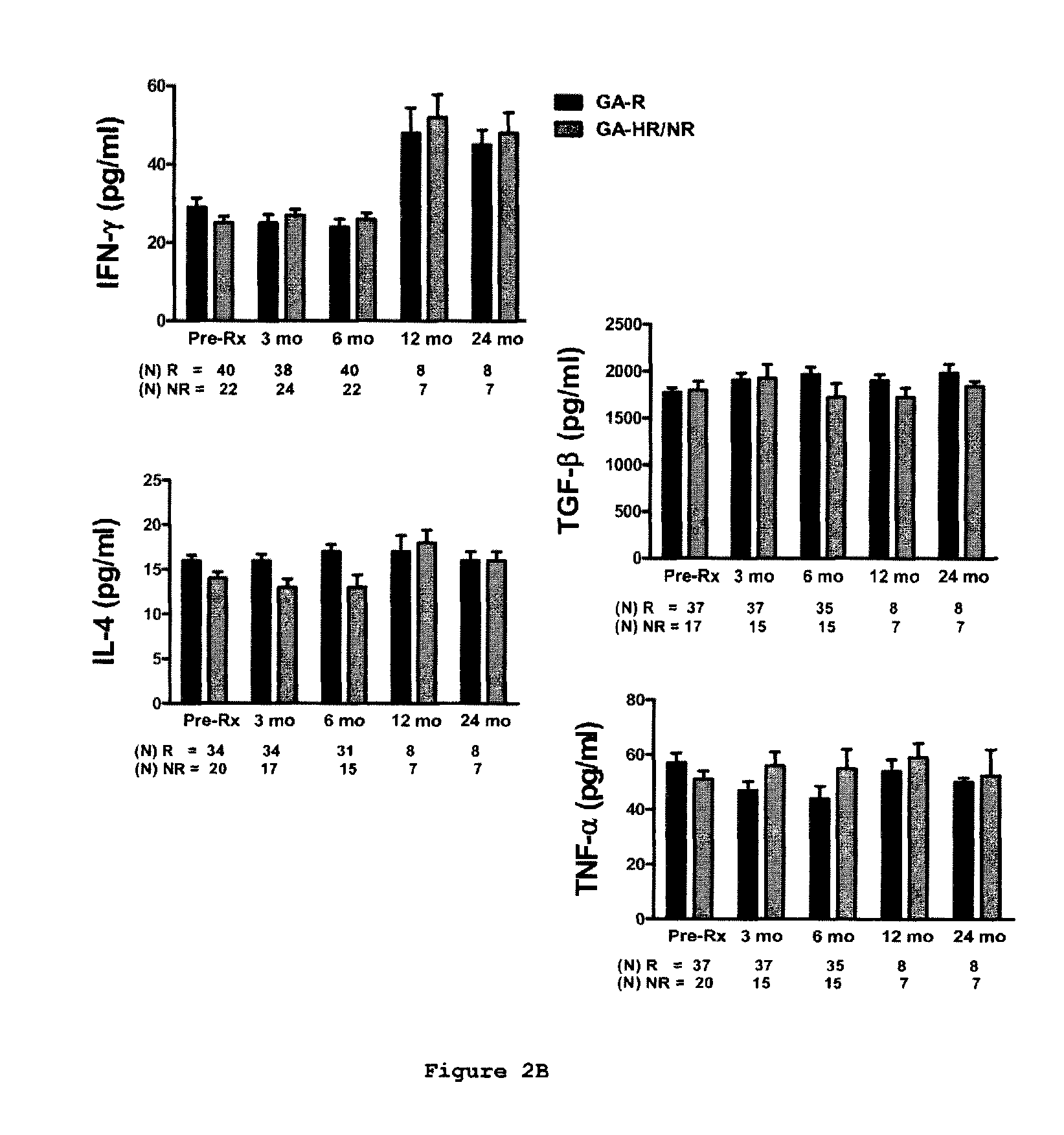
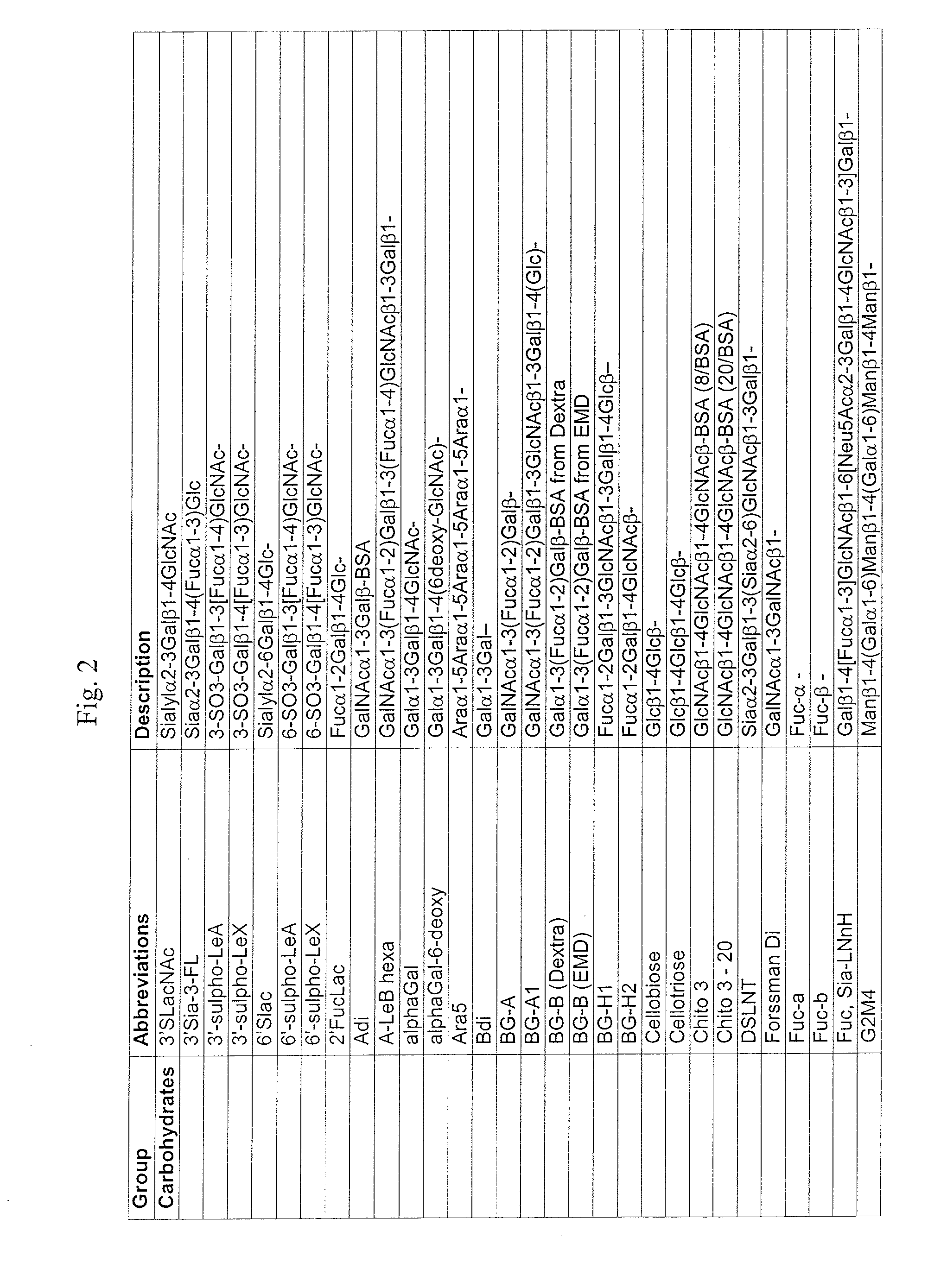
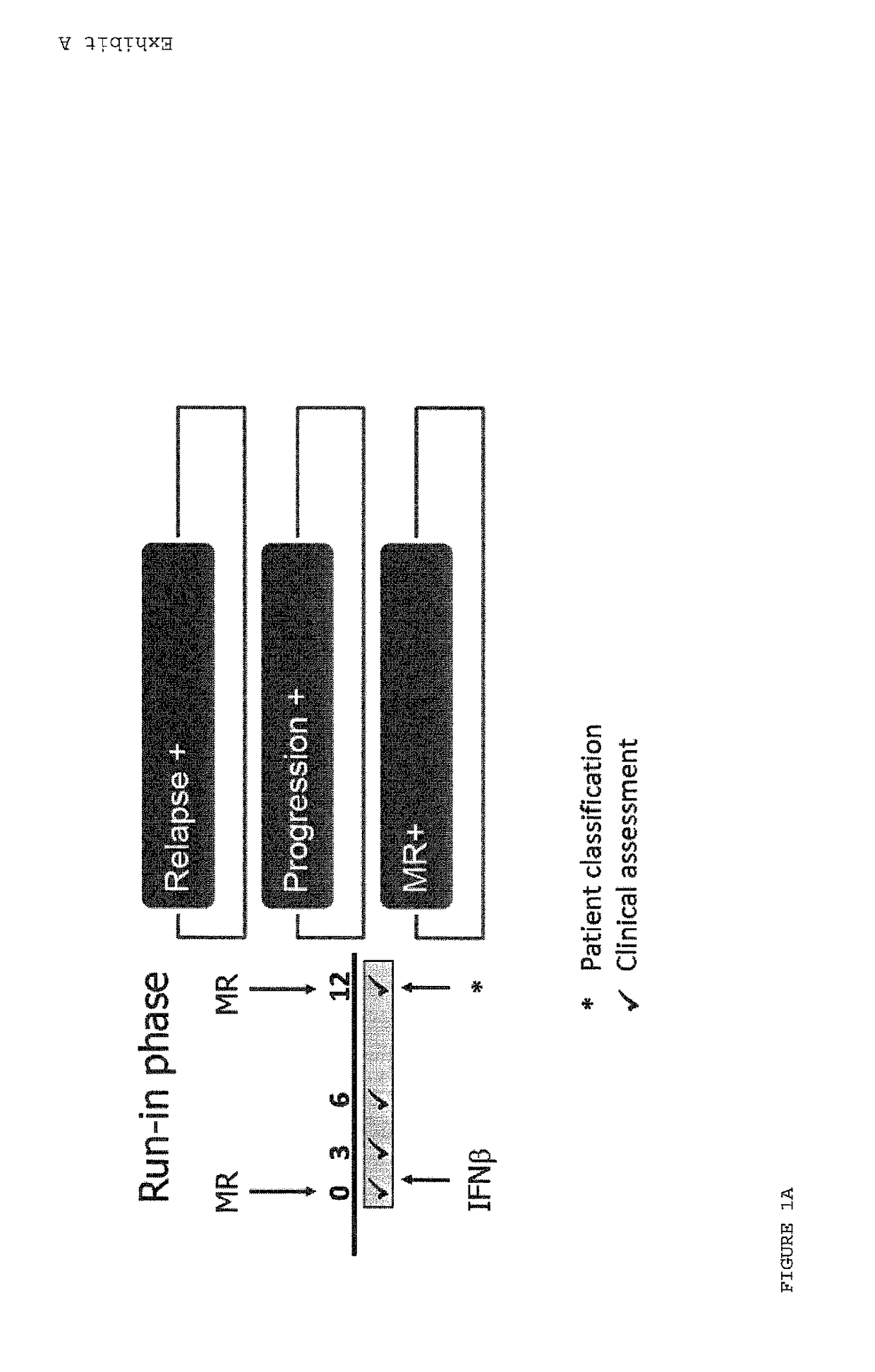
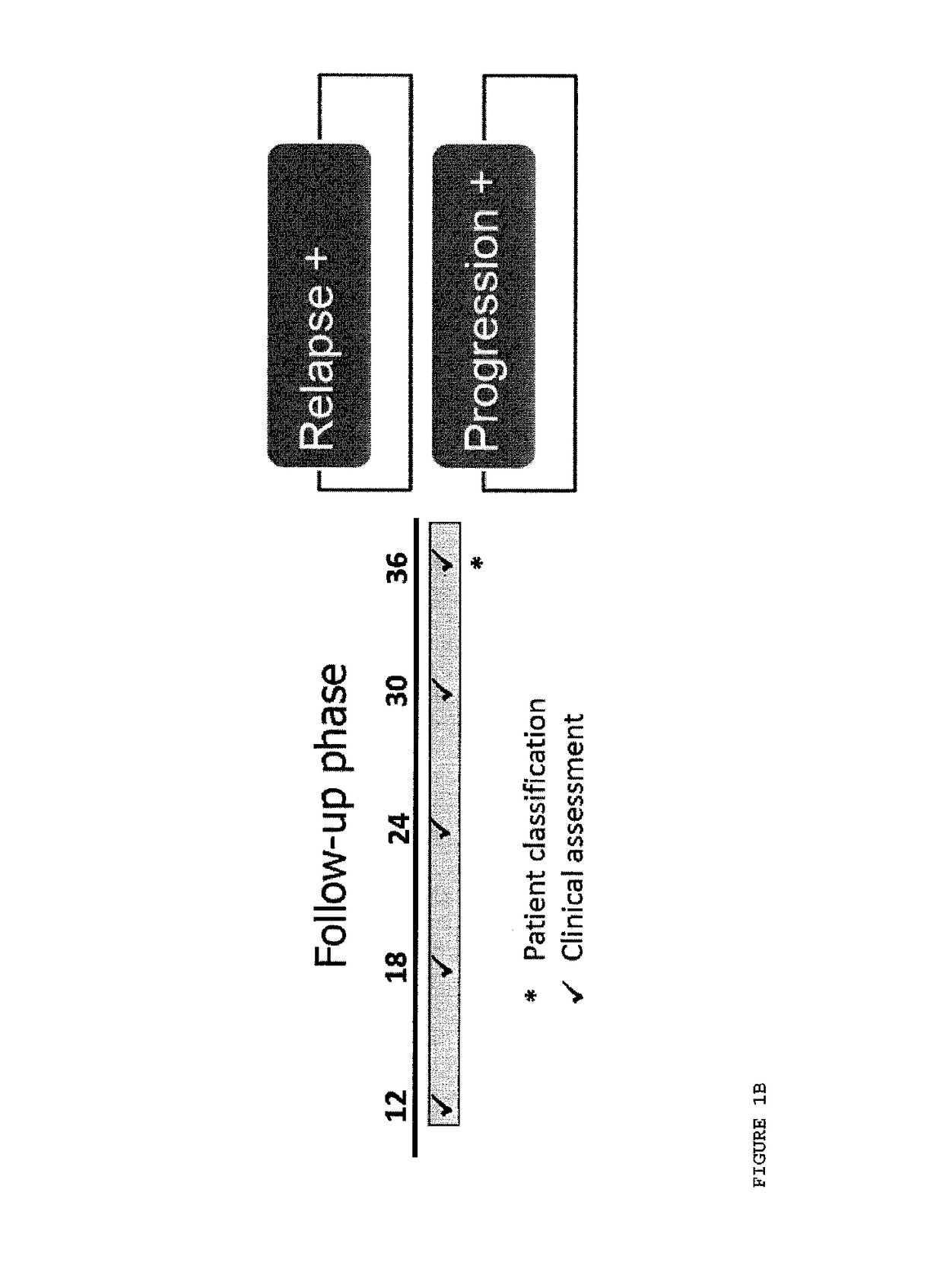
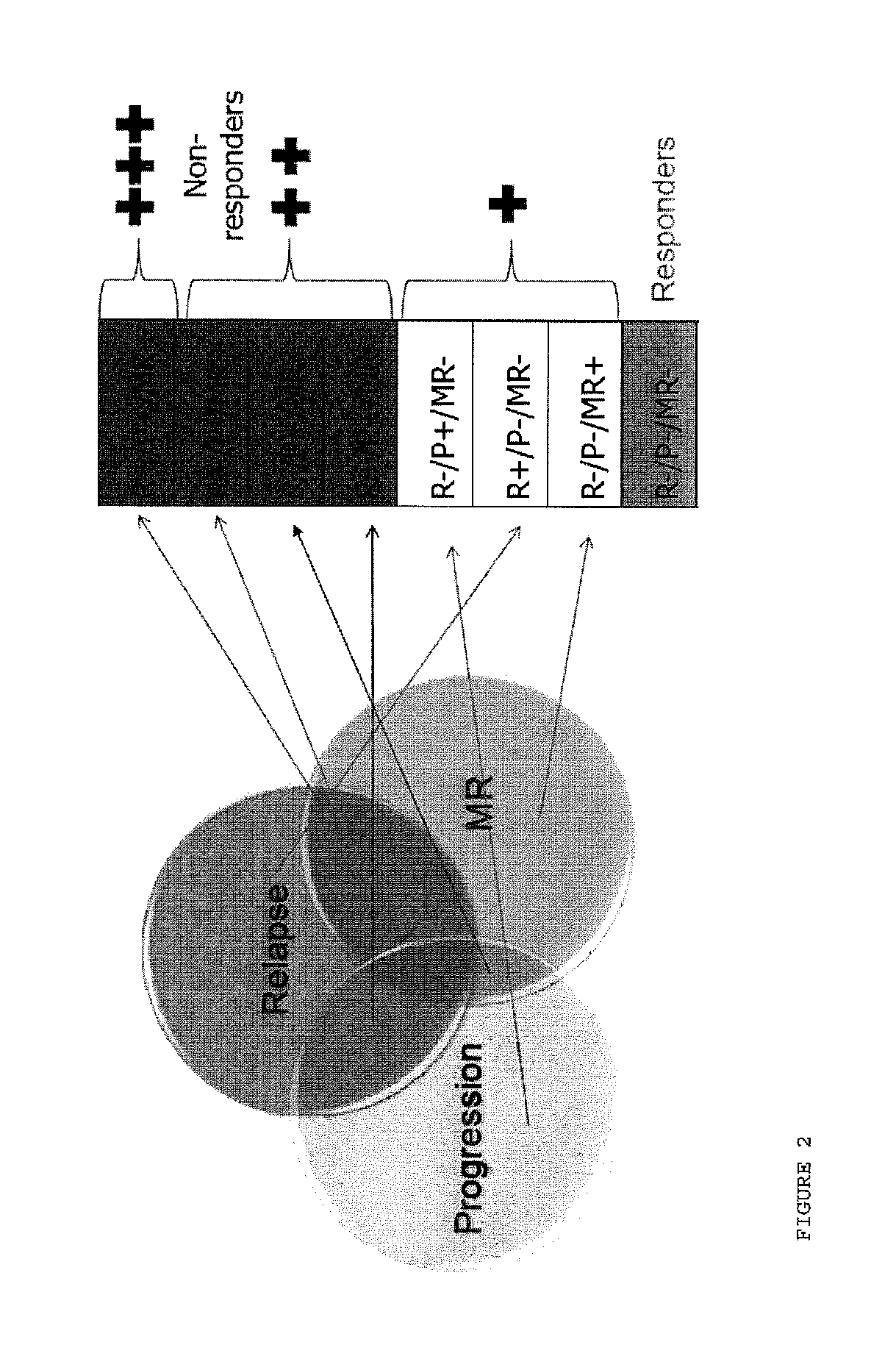
Methods of treating a subject afflicted with an autoimmune disease using predictive biomarkers of clinical response to glatiramer acetate therapy in multiple sclerosis
Owner:TEVA PHARMA IND LTD
Methods and reagents for decreasing clinical reaction to allergy
InactiveUS20070213507A1Less allergicEliminate IgE bindingPeptide/protein ingredientsFungi peptidesBinding siteT cell
It has been determined that allergens, which are characterized by both humoral (IgE) and cellular (T cell) binding sites, can be modified to be less allergenic by modifying the IgE binding sites. The IgE binding sites can be converted to non-IgE binding sites by masking the site with a compound that prevents IgE binding or by altering as little as a single amino acid within the protein, most typically a hydrophobic residue towards the center of the IgE-binding epitope, to eliminate IgE binding. The method allows the protein to be altered as minimally as possible, other than-within the IgE-binding sites, while retaining the ability of the protein to activate T cells, and, in some embodiments by not significantly altering or decreasing IgG binding capacity The examples use peanut allergens to demonstrate alteration of IgE binding sites. The critical amino acids within each of the IgE binding epitopes of the peanut protein that are important to immunoglobulin binding have been determined. Substitution of even a single amino acid within each of the epitopes led to loss of IgE binding. Although the epitopes shared no common amino acid sequence motif, the hydrophobic residues located in the center of the epitope appeared to be most critical to IgE binding.
Owner:MT SINAI SCHOOL OF MEDICINE +1
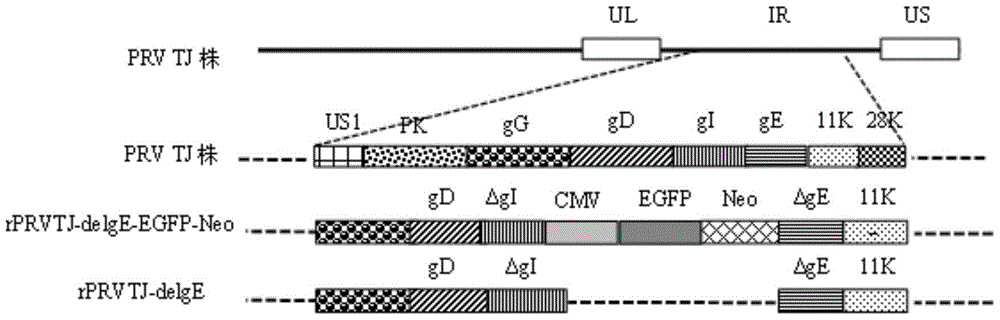
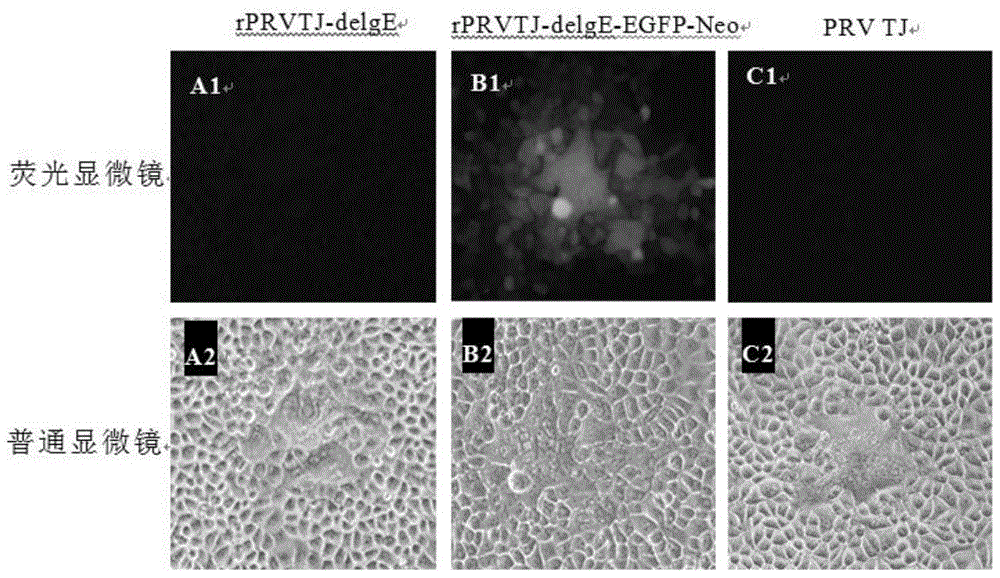
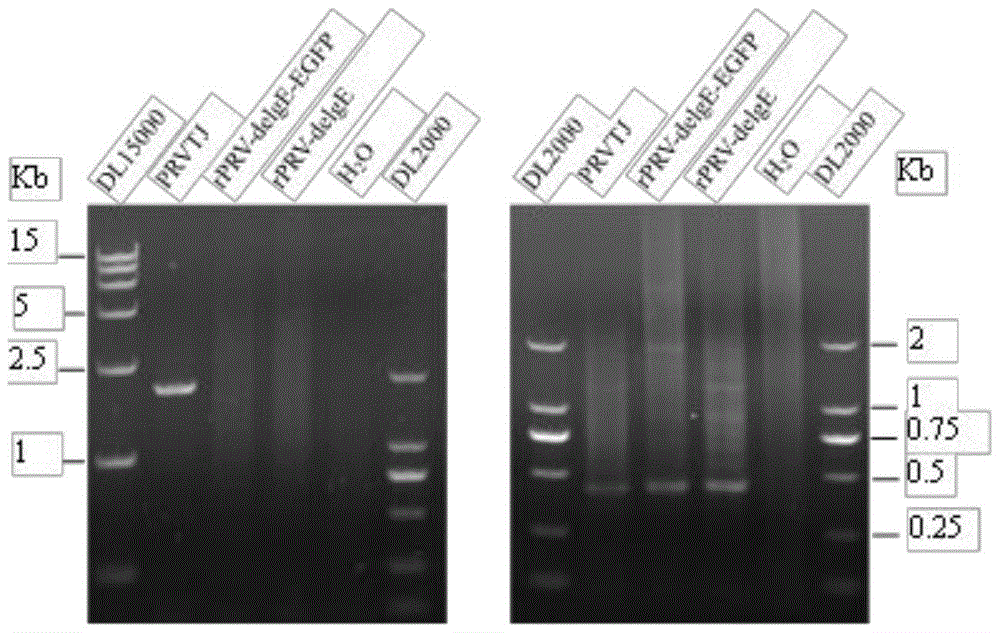
Double gene-deleted strain of pseudorabies virus variant, construction method and application thereof
ActiveCN104059889AImprove purification efficiencyImprove screening efficiencyInactivation/attenuationMicroorganism based processesEnzyme digestionTransfer vector
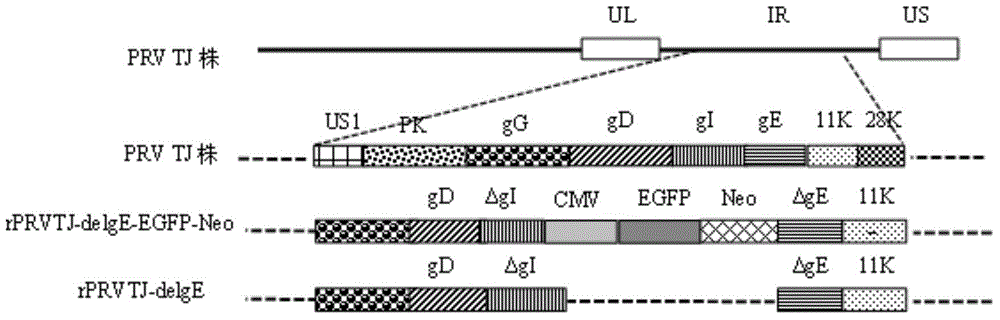
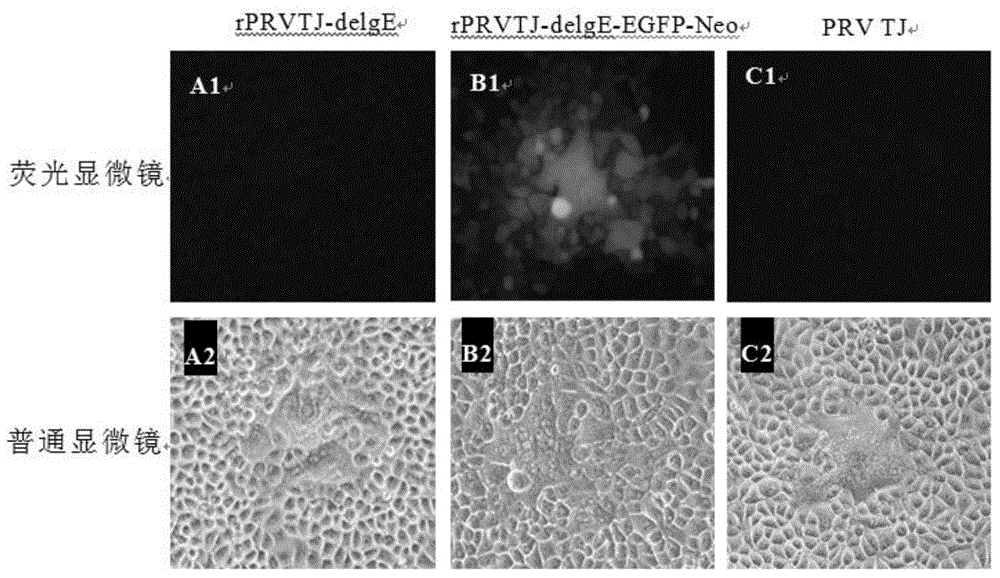
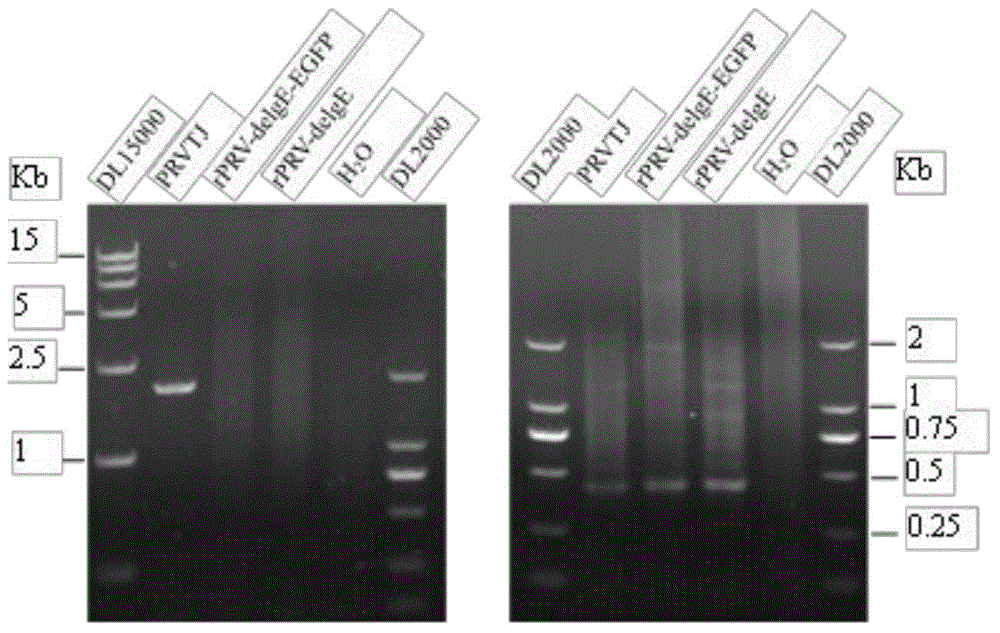
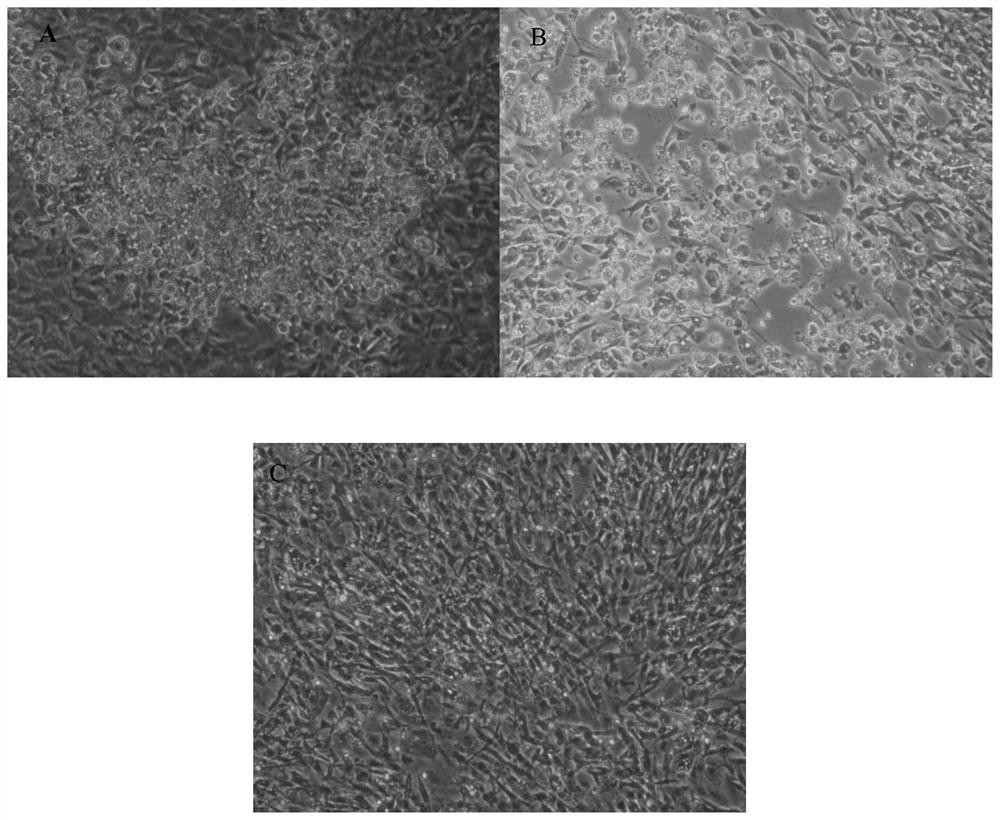
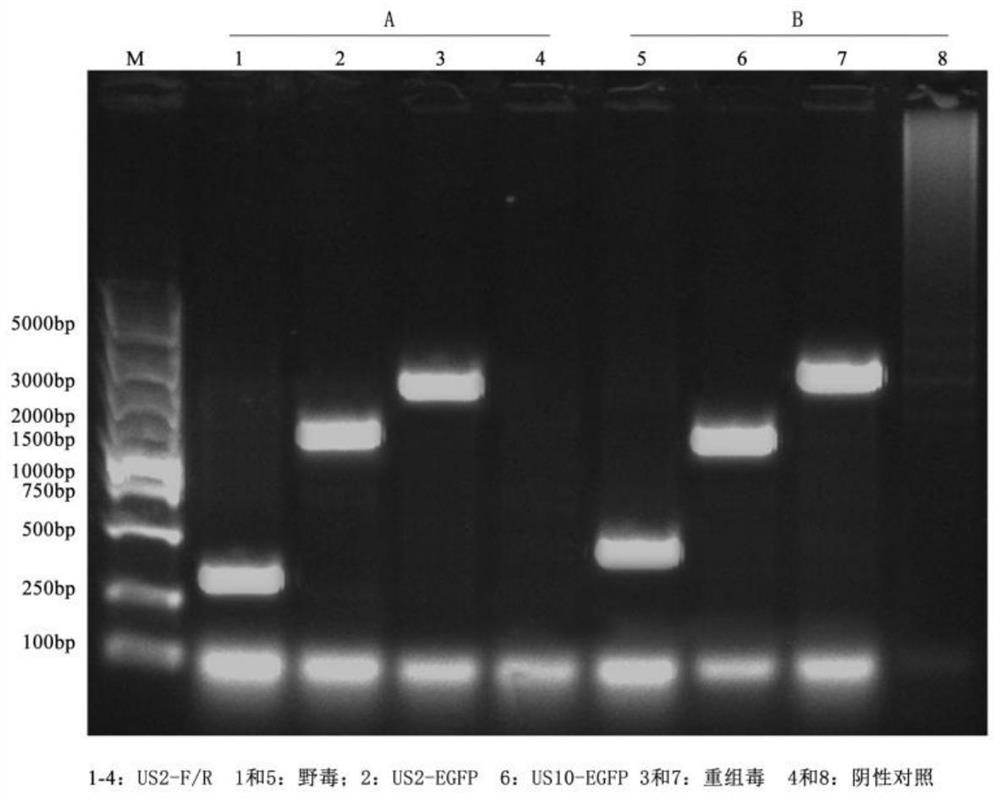
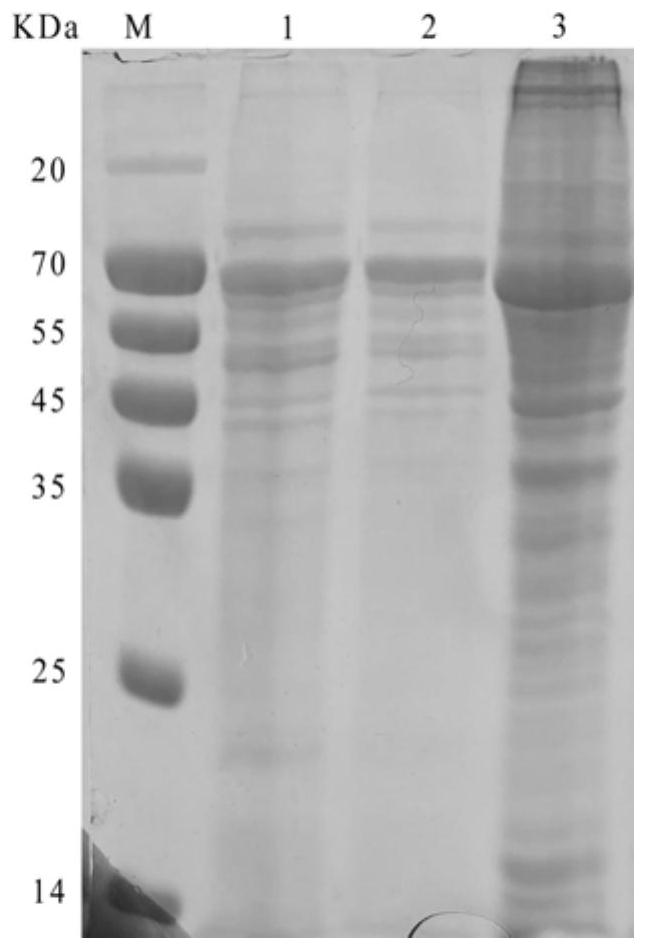
The invention discloses a double gene-deleted strain of a pseudorabies virus variant, a construction method and an application thereof. The invention provides the pseudorabies virus strain with gI and gE genes being deleted. The pseudorabies virus variant is assigned the access number CGMCC. NO.8786. The invention further provides the construction method for the double gene-deleted strain. The construction method includes: (1) constructing a pseudorabies virus TJ strain transfer vector containing complete expression cassettes of EGFP and Neo; (2) transfecting the transfer vector to a cell which is inoculated with a pseudorabies virus TJ strain to obtain a transitional virus; and (3) performing an enzyme digestion process to a transitional viral genome, co-transfecting the enzyme digested transitional viral genome with the transfer vector and performing a plague screening process to obtain the double gene-deleted strain. The double gene-deleted strain is completely attenuated, is free of any clinical reaction after immunization of pigs, can rapidly induce generation of a specific antibody of pseudorabies virus, is high in neutralizing antibody titer and can provides a complete immune protection which aims at an attack of a presently-epidemic pseudorabies virus variant.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Medium-chain and long-chain fat emulsion injection and preparation method thereof
ActiveCN103505415ADownsides of Too Much Omega-6 Fatty AcidsImprove the level ofOrganic active ingredientsMetabolism disorderAntioxidantFiltration
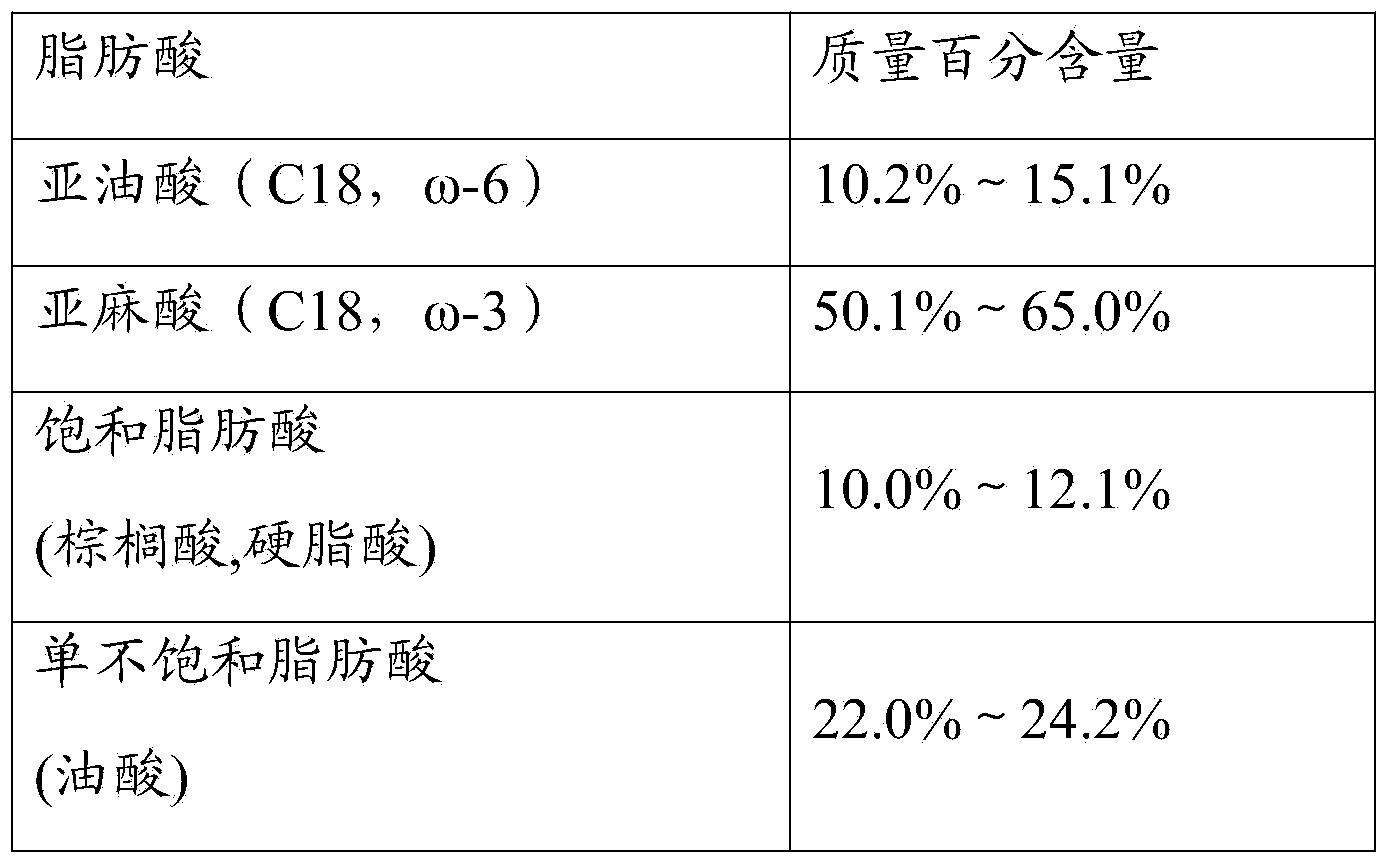
The invention discloses medium-chain and long-chain fat emulsion injection and a preparation method thereof. The injection is prepared by the following components: every 1000 ml of injection contains 50-200 g of refined linseed oil, 50-200 g of medium-chain oil for injection, 6-18 g of an emulsifier, 15-35 g of an isoosmotic adjusting agent, 0.1-2 g of an antioxidant, and water for injection in balancing amount, wherein the pH value of the injection is adjusted to be 5.0-9.0 by adopting a pH value conditioning agent, and the medium-chain oil for injection is medium-chain triglyceride. The preparation method comprises oil phase preparation, water phase preparation, elementary fat emulsion preparation, homogenization and fine filtration. The medium-chain and long-chain fat emulsion injection provided by the invention is a fat emulsion for injection containing the linseed oil, and has good stability; due to the low content of linoleic acid and the high content of linolenic acid in the medium-chain and long-chain fat emulsion injection, the adverse clinical reactions caused by high content of the linoleic acid in the existing fat emulsion injection are solved; and the medium-chain and long-chain fat emulsion injection has the important efficacy of maintaining lipoprotein balance, reducing blood fat, regulating cholesterol metabolism, dropping blood pressure, resisting thrombus and preventing canceration.
Owner:HUAREN PHARMACEUTICAL CO LTD
Immune Biomarkers and Assays Predictive of Clinical Response to Immunotherapy for Cancer
The present invention relates to predictors of a cancer patient's responsiveness to immunotherapy for cancer.
Owner:YALE UNIV
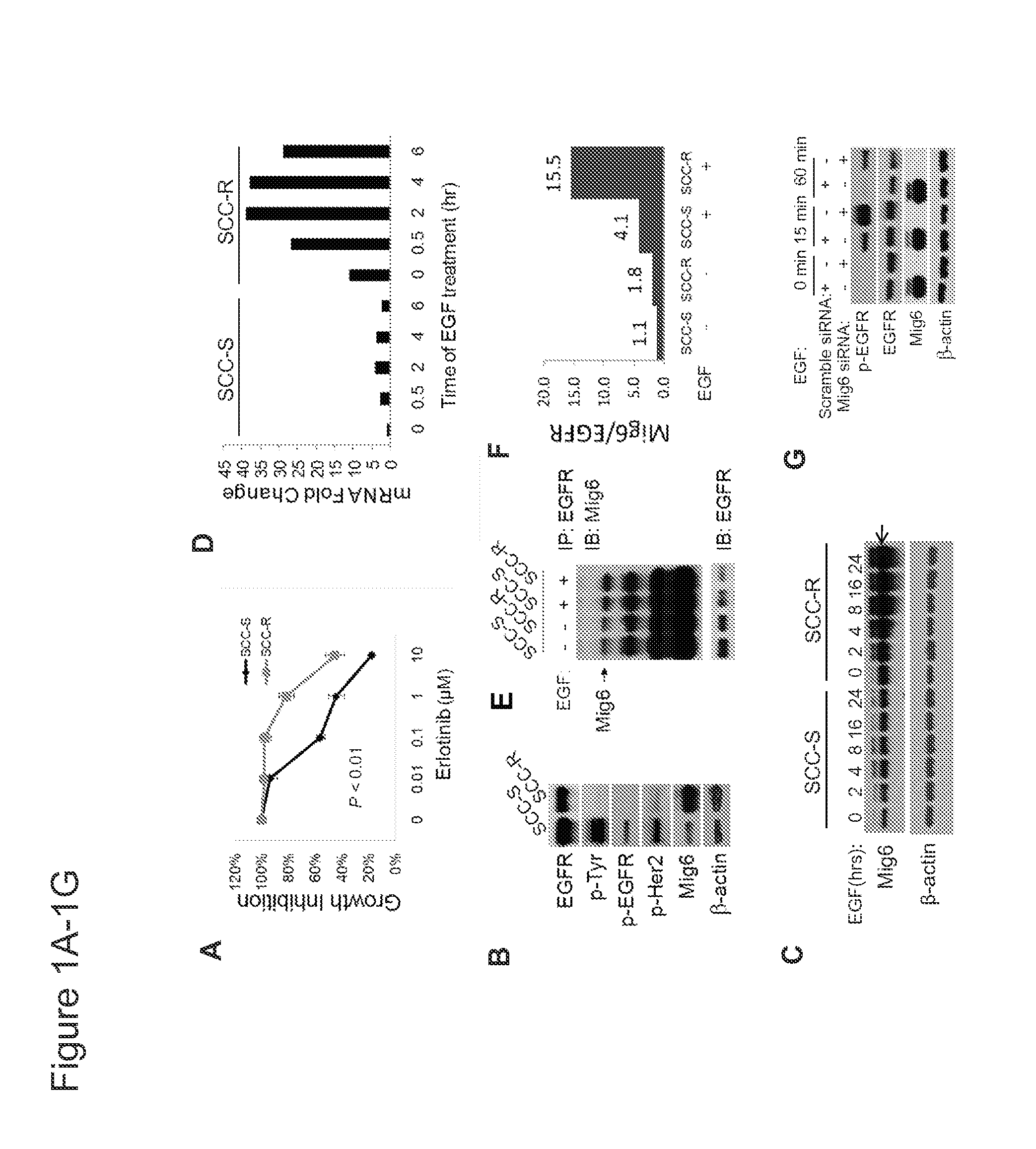
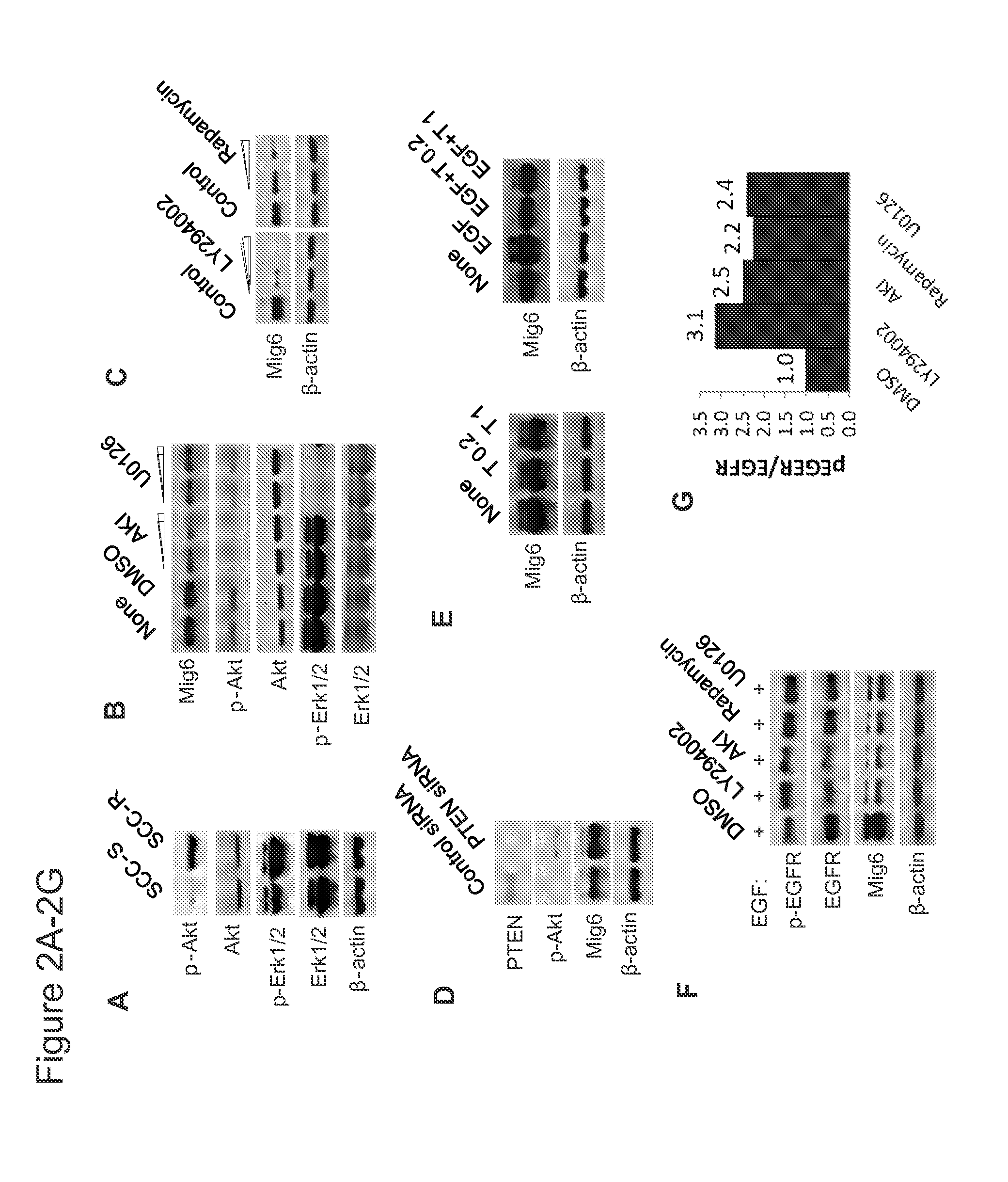
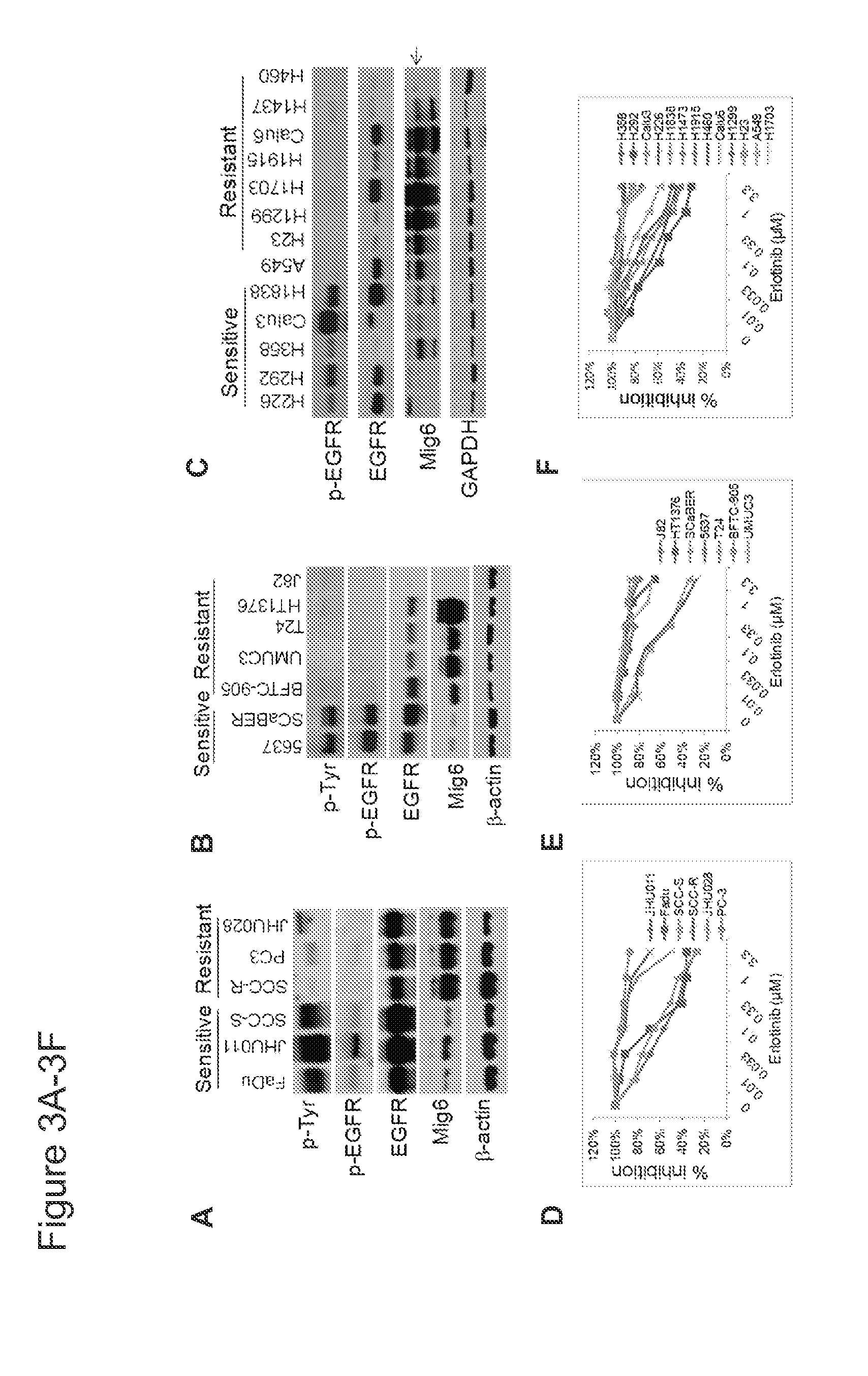
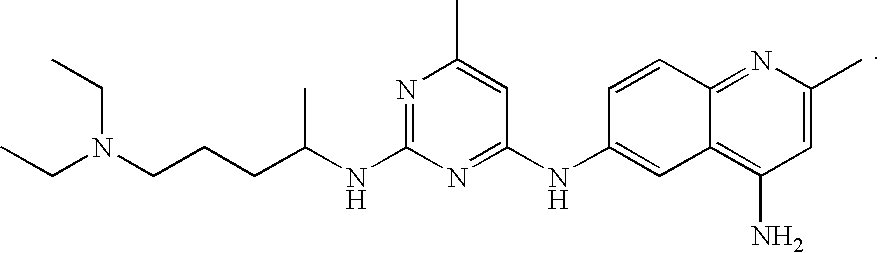
Mig6 and therapeutic efficacy
We identify markers capable of guiding the decision to incorporate epidermal growth factor receptor (EGFR) inhibitors, in particular EGFR tyrosine kinase inhibitors (TKIs), into chemotherapeutic regimens. Mitogen-inducible gene 6 (Mig6), a negative regulator of EGFR, is selectively upregulated during the development of resistance to the EGFR tyrosine kinase inhibitor (TKI) erlotinib, resulting in decreased EGFR phosphorylation. The ratio of Mig6 / EGFR expression highly correlates with erlotinib sensitivity. A low Mig6 / EGFR ratio correlates with a high response rate to gefitinib and a marked increase in progression-free survival for patients. The ratio of Mig6 to EGFR is a major predictor of biologic and clinical responses to EGFR inhibitors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
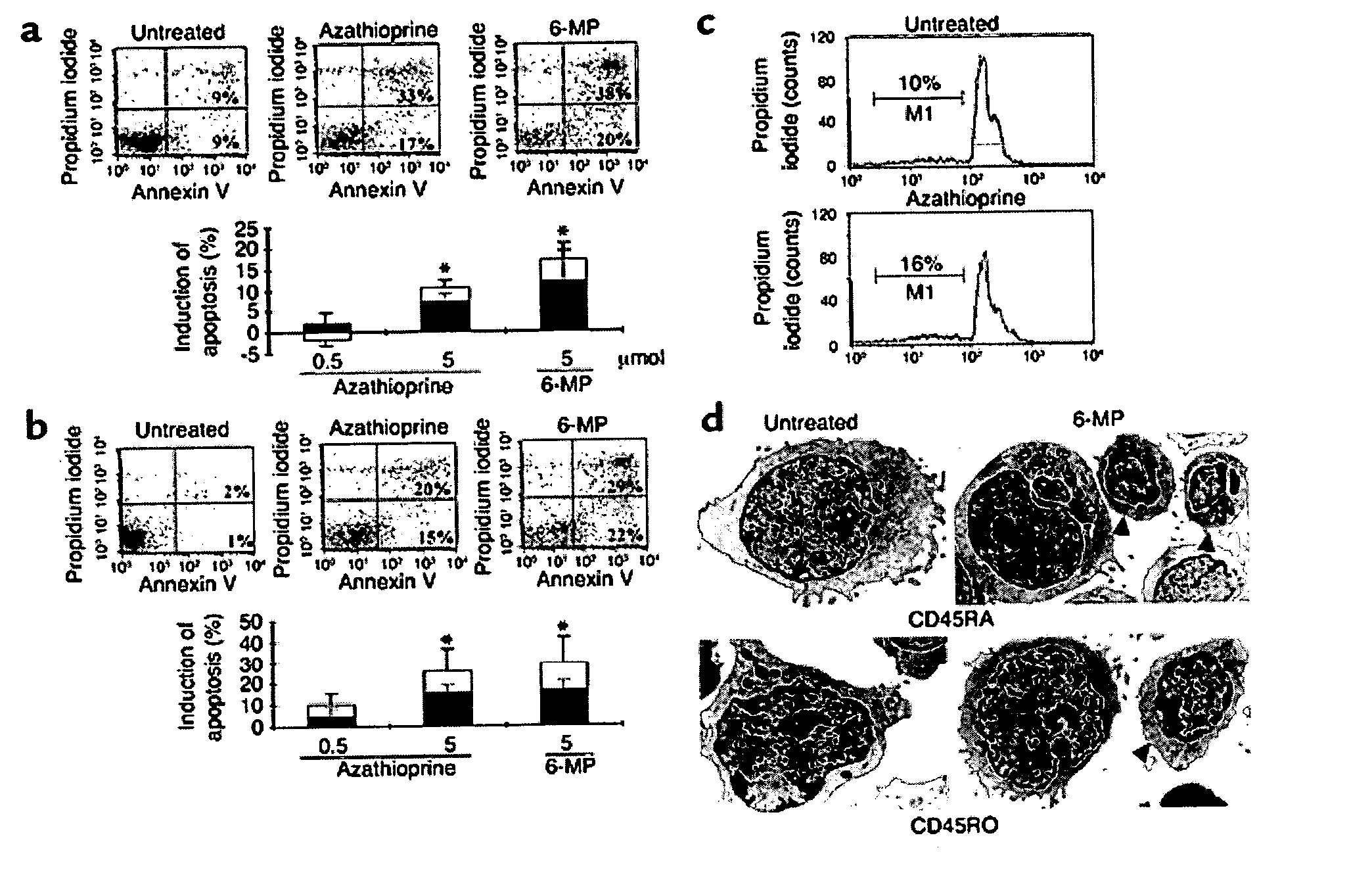
Diagnostic methods for therapeutic compounds and methods for monitoring azathioprine therapy
The present invention provides diagnostic methods for predicting therapeutic efficacy in an individual being treated for an autoimmune or an inflammatory disease. In addition, the present invention also provides novel methods for monitoring azathioprine therapy or optimizing clinical responsiveness to azathioprine therapy in an individual by measuring 6-thioguanosine nucleotide levels in a sample from the individual.
Owner:ROBERT BOSCH GES FUR MEDIZINISCHE FORSCHUNG RBMF
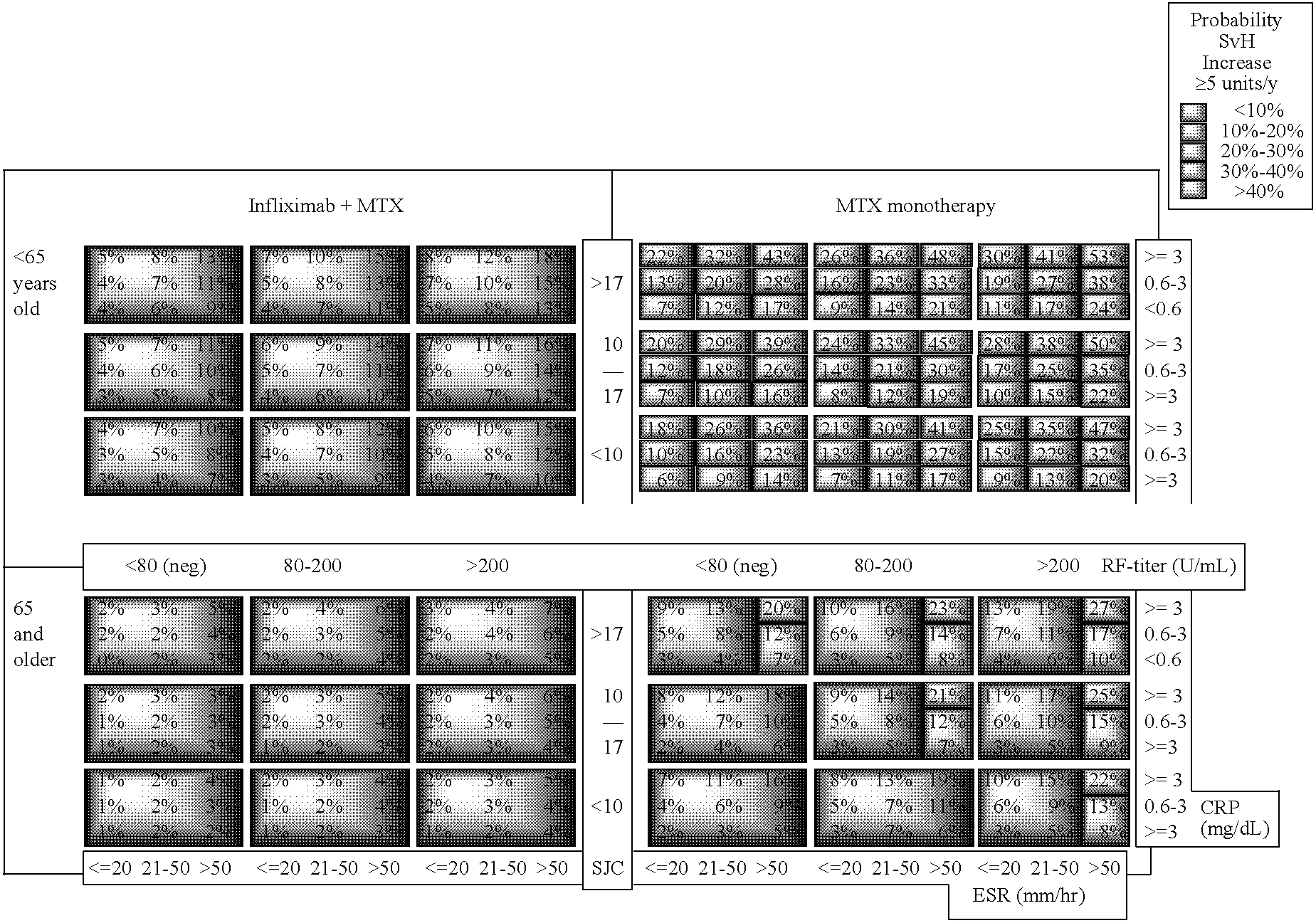
Matrix marker model and methods for assessing and treating arthritis and related disorders
The invention relates to a method of determining the efficacy of the treatment for RA based on two to several characteristics known to correlate with negative outcomes in RA presented in a matrix, as well as a method of determining the efficacy of the treatment for RA based on preparing matrix table comprising two to several covariates which significantly correlate with radiological progression, where the matrix relates this profile of characteristics or covariates to the probability (risk) of negative outcomes under each of the alternative potential treatments, which can be done for a subject, for example, prior to the manifestation of other gross measurements of clinical response.
Owner:HAN CHENGLONG +2
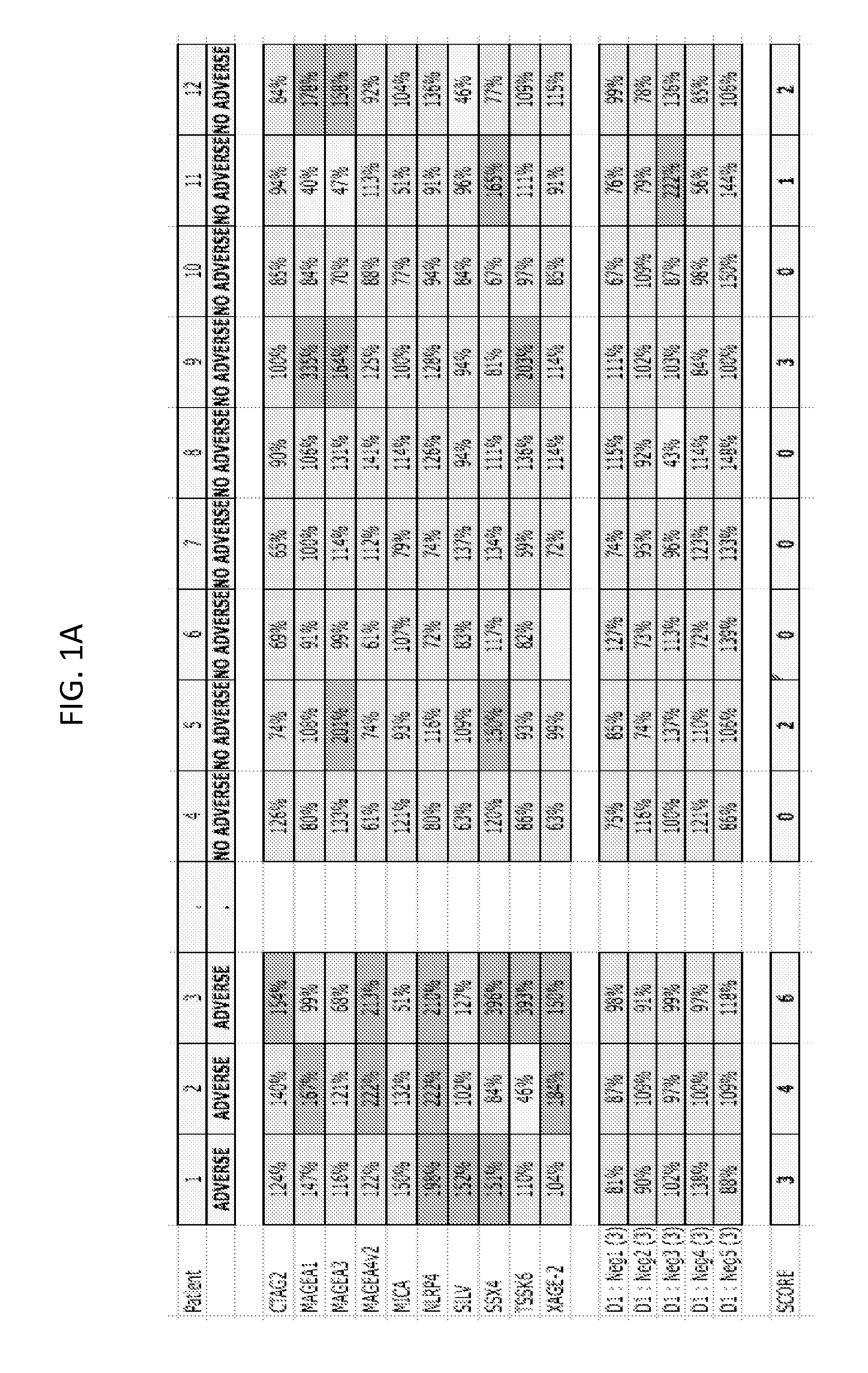
Materials and methods for differential treatment of cancer
InactiveUS20150044224A1Treating and delaying onset and relapsePeptide librariesNucleotide librariesEfficacyOncology
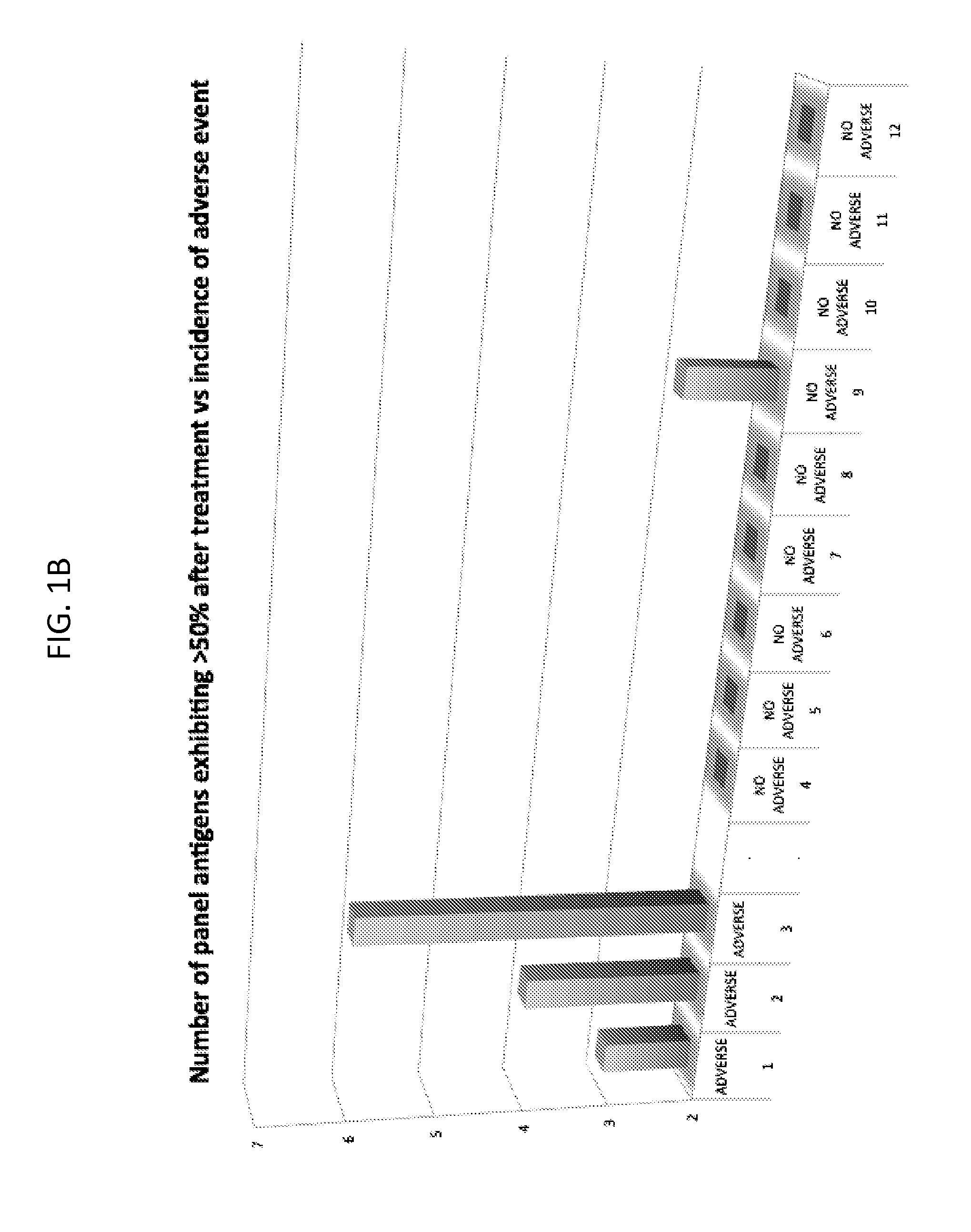
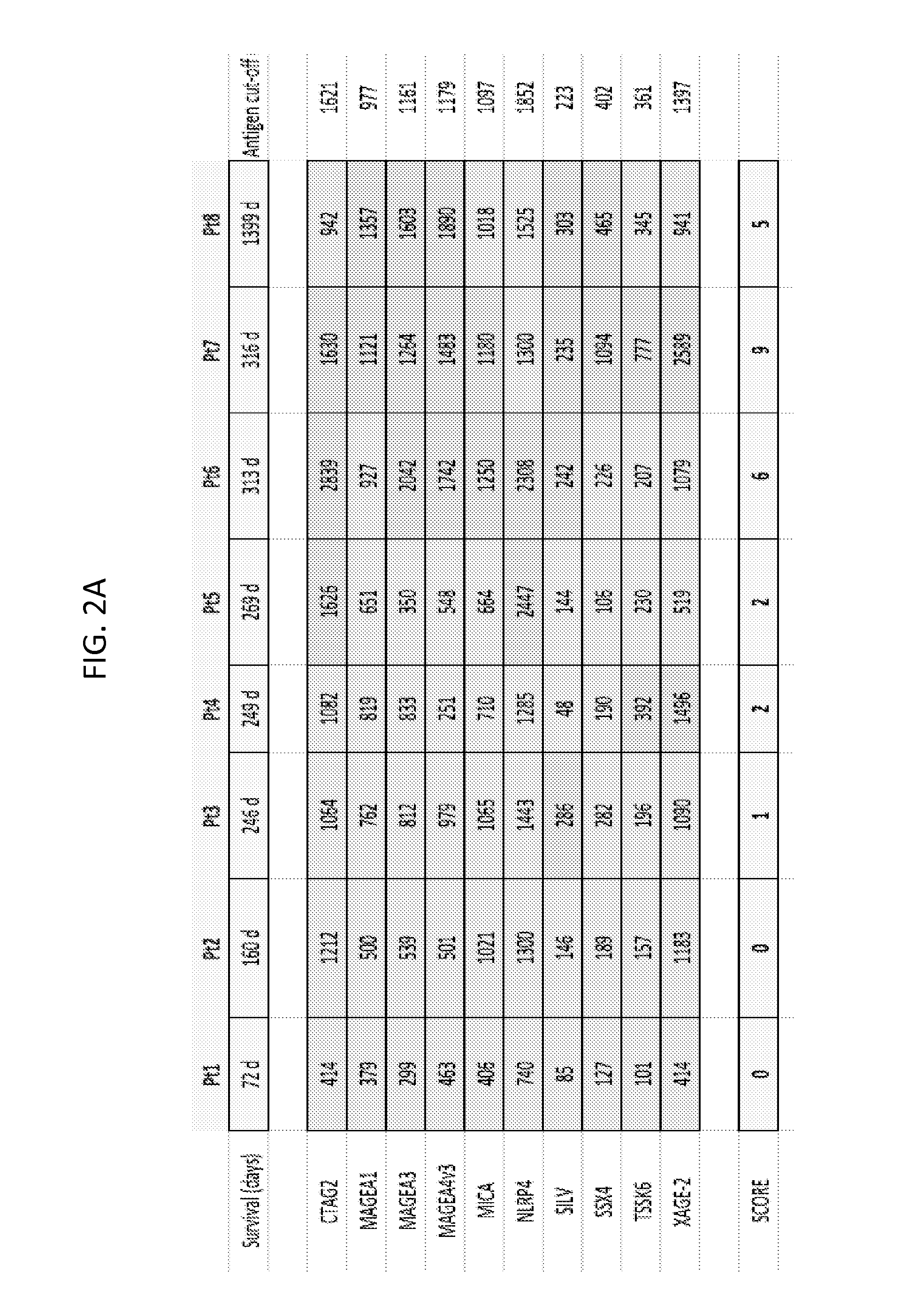
The present invention concerns differential therapeutic treatment of cancer patients based on prognostic antigen / antibody profiles used for predicting (prognosticating) a clinical response (efficacy) and / or adverse event to an immunotherapy for treatment of a malignancy in a subject, and for treating or delaying the onset or relapse of a malignancy in a subject.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC +1
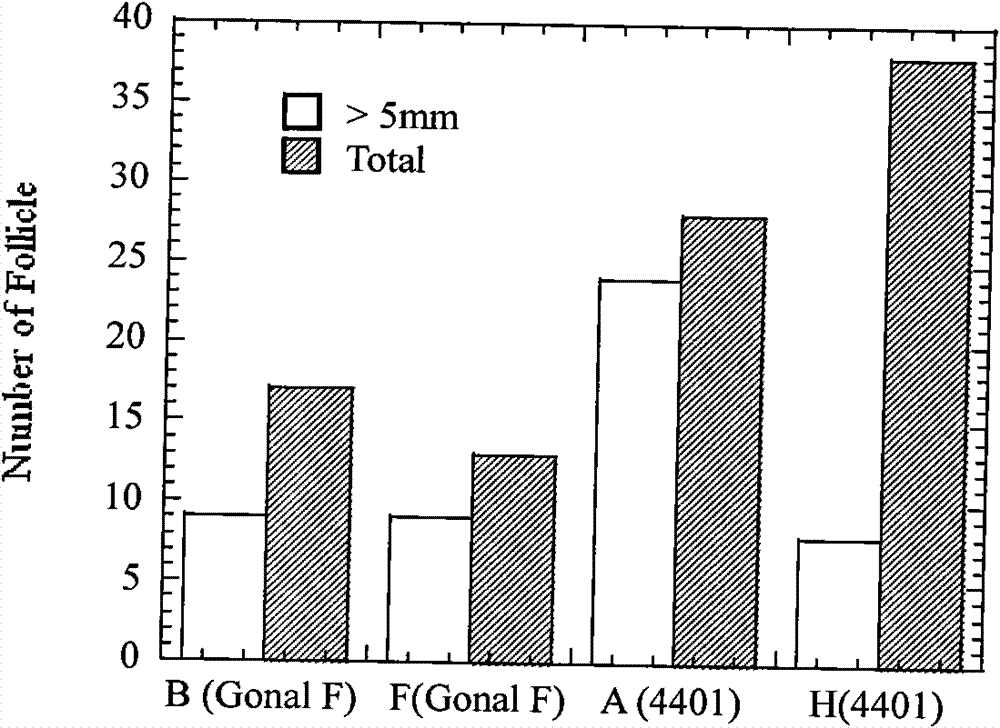
Preparation method for rhesus ovary and ovarian follicle generation model
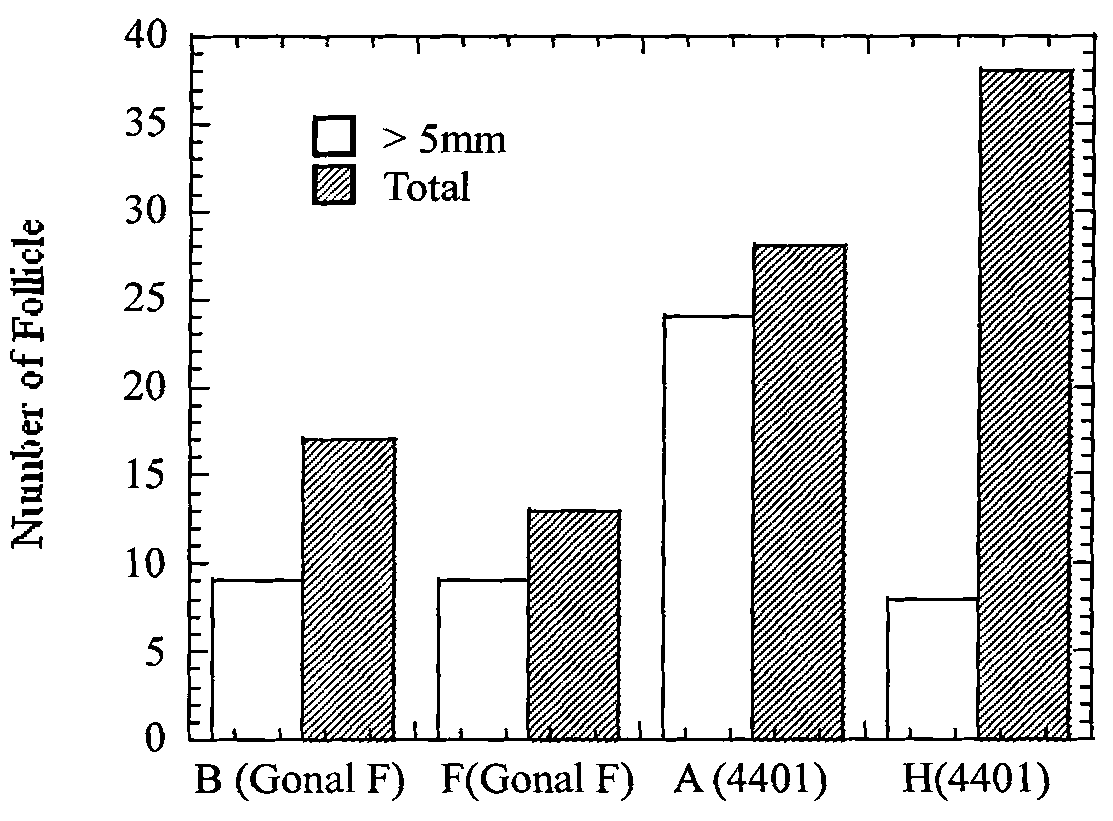
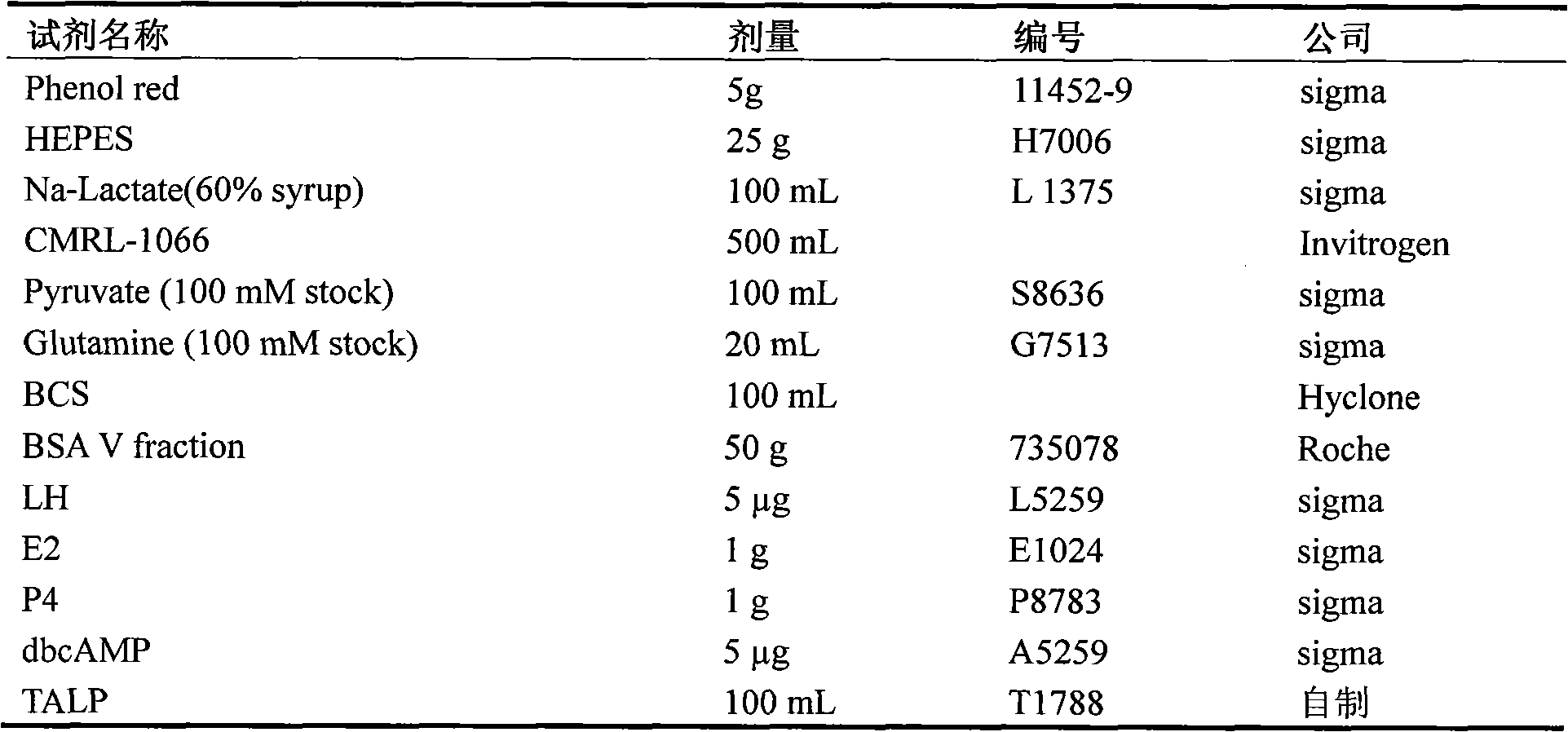
ActiveCN102370973AFunction and effect are indeedAction and effect are stablePeptide/protein ingredientsSexual disorderMedicine.hematologyPhysiology
The invention provides a preparation method for a rhesus ovary and ovarian follicle generation model. According to the method, female rhesus monkeys are used as test objects, and each rhesus monkey is injected with 60 IU of luteinizing hormone LH each time and is injected twice everyday for seven days successively to prepare the rhesus ovary and ovarian follicle generation model. The invention also provides application of luteinizing hormone LH in preparing medicines for activating ovary and stimulating ovarian follicle thereof to grow. According to the invention, monkeys are used in the method for preparing the model, background data in physiology, hematology, blood biochemistry and the like of monkeys are abundant and easily available, monkeys have a biological background resembling to human beings, and therefore, test results of monkeys can well indicate clinical reactions of medicines; multi-aspect and multi-angle assessment are carried out, and obtained results can well and objectively indicate drug action on a reproductive system; animals (monkeys) and instruments (a B-mode ultrasonoscope, a microscope, a cell culture device, etc.) used in the model are simple and easily available, and the model has versatility.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +2
Traditional Chinese medicine fumigation lotion for treating achilles tendonitis and preparation method thereof
InactiveCN106237188AGood curative effectStrong muscleHydroxy compound active ingredientsAntipyreticDiseaseSide effect
The invention discloses a traditional Chinese medicine fumigation lotion for treating achilles tendonitis and a preparation method thereof, and belongs to the field of traditional Chinese medicines. The active components of the medicine provided by the invention comprise the following raw materials: radix et folium psychotriae serpentis, radix et caulis ventilaginis leiocarpae, radix uncariae rhynchophyllae, radix salviae miltiorrhizae, elatostema stewardii merr, caulis kadsurae, herba goodyerae, rhizoma polygoni cuspidati, herba ainsliaea albo-tomentosae, herba tetrastigmatis obtecti, cortex acanthopanacis, radix glycyrrhizae, radix seu caulis kadsurae heteroclitae, cortex schefflerae octophyllae, radix cynoglossi amabilis, rhizoma ligustici chuanxiong, fructus foeniculi, dioscorea tenuipes, daphne koreana nakai, cinnamomum tamala, wikstroemia canescens, rhizoma et radix smilacis nipponicae, radix et folium murrayae and borneolum syntheticum. The traditional Chinese medicine fumigation lotion selects medicinal materials conforming to the principles of monarch, minister, assistant and guide, is applied according to cause of disease, has effects of dispelling wind and eliminating dampness, warming the meridians and dredging blood vessel, promoting blood circulation to remove blood stasis, promoting qi to activate blood circulation, reducing swelling and resolving mass, relaxing tendons and activating collaterals, strengthening the muscles and bones, and promoting circulation of qi to relieve pain, can effectively relieve pain, promotes blood circulation, accelerate tissue repair, has good absorption effect, obvious treatment effect, no toxic and side effects and no adverse clinical reactions, and is clinically proven to have high cure rate for achilles tendonitis.
Owner:QINGDAO ZHIXIN TIANCHENG TECH DEV CO LTD
Methods and reagents for decreasing clinical reaction to allergy
InactiveUS7879977B2Less allergicEliminate IgE bindingPeptide/protein ingredientsProtein composition from yeastsBinding siteADAMTS Proteins
It has been determined that allergens, which are characterized by both humoral (IgE) and cellular (T cell) binding sites, can be modified to be less allergenic by modifying the IgE binding sites. The IgE binding sites can be converted to non-IgE binding sites by masking the site with a compound that prevents IgE binding or by altering as little as a single amino acid within the protein, most typically a hydrophobic residue towards the center of the IgE-binding epitope, to eliminate IgE binding. The method allows the protein to be altered as minimally as possible, other than-within the IgE-binding sites, while retaining the ability of the protein to activate T cells, and, in some embodiments by not significantly altering or decreasing IgG binding capacity The examples use peanut allergens to demonstrate alteration of IgE binding sites. The critical amino acids within each of the IgE binding epitopes of the peanut protein that are important to immunoglobulin binding have been determined. Substitution of even a single amino acid within each of the epitopes led to loss of IgE binding. Although the epitopes shared no common amino acid sequence motif, the hydrophobic residues located in the center of the epitope appeared to be most critical to IgE binding.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Pill for treating chronic lymphocytic thyroiditis and preparation method
InactiveCN104491364AHigh cure ratePromote absorptionInorganic active ingredientsDrug compositionsOysterAllium macrostemon
The invention discloses a pill for treating chronic lymphocytic thyroiditis and a preparation method, belonging to the field of traditional Chinese medicines. The effective components of the pill disclosed by the invention are composed of the following raw materials: 65-80 g of Chinese yam, 63-76 g of radix pseudostellariae, 60-73 g of immature bitter orange, 57-70 g of wolfberry, 55-67 g of semen litchi, 53-65 g of radix bupleuri, 50-62 g of white atractylodes rhizome, 43-55 g of the root of three-nerved spicebush, 40-52 g of glossy privet fruit, 38-50 g of stellaria aquatica, 35-47 g of tendril-leaved fritillary bulb, 32-45 g of pericarpium citri reticulatae viride, 30-42 g of fructus forsythiae, 28-40 g of rhizoma atractylodis, 25-37 g of liquorice, 23-35 g of allium macrostemon, 20-33 g of rose, 18-30 g of fingered citron, 15-28 g of selfheal, 12-25 g of sea horse, 9-22 g of oyster, 6-15 g of turtle shell, 4-10 g of pumex, and 2-7 g of air potato. The selected medicinal materials in the invention accord with monarch, ministerial and adjuvant principles; aiming at pathogenesis, treatment is carried out according to different symptoms; both symptoms and root causes are treated; the pill disclosed by the invention has the effects of tonifying the spleen and kidney, promoting blood circulation to remove blood stasis and resolving hard lump, and has the advantages of being good in absorption effect, obvious in curative effect and free from toxic and side effects and poor in clinical reaction; and in addition, found from clinical verifications, the pill disclosed by the invention has higher curative rate to chronic lymphocytic thyroiditis.
Owner:武威天健药业有限公司
Method for predicting and monitoring clinical response to immunomodulatory therapy
ActiveUS11175282B2Reduce riskReduce adverse side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsCell culture supports/coatingIMMUNE SUPPRESSANTSSide effect
The present invention provides a method to quantitatively measure the response of a patient to an immune-modulator drug that will aid clinicians in the determination of the optimal combination / posology of immunosuppressant / immune-modulator drugs. In addition, this method will open the possibility for clinicians to make the necessary adjustments in immunosuppressive therapy, as a way to avoid organ rejection to actually take place. Furthermore, this method will significantly reduce side effects of immunosuppressant drugs, optimizing therapeutic scheme and dosages, enabling the determination of the most effective immunosuppression regimen at the lower dosages for each patient individually and monitoring of treatment efficiency along time, thus opening the door to treatment personalization.
Owner:BIOHOPE SCI SOLUTIONS FOR HUMAN HEALTH SL
Predicting responses to immunotherapy
PendingCN111344568AImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisDiseaseImmunomodulating Agent
The invention relates to methods for determining the likelihood of the formation of a clinical response in an individual to therapy with an immunomodulatory agent for treatment of a disease or condition of the individual.
Owner:IMMUNESIGNATURES PTY LTD
Traditional Chinese medicine electuary for treating tourette syndrome of children and preparation method
InactiveCN105596697AGood curative effectHigh cure rateNervous disorderHydroxy compound active ingredientsMedicinal herbsLiver and kidney
The invention discloses a traditional Chinese medicine electuary for treating the tourette syndrome of children and a preparation method and belongs to the field of traditional Chinese medicine. Effective constituents of the medicine include roots of ficus beecheyana, roots of rehmannia, rust-colored crotalaria herb with roots, turtle shells, rhizomes of perny false fairybells, liquorice roots, tuber fleeceflower stems, rose flowers, fruits of Chinese wolfberry, radix angelica sinensis, semen cuscutae, radix pseudostellariae, alectoria asatica Du Rietz, radix bupleuri, horns of procapra gutturosa pallas, arisaema cum bile, folium apocyni veneti, oysters, fresh bulbs of fritillary, fructus gardenia, irkutsk anemone rhizomes, manyleaf paris rhizomes, autumn zephyrlily herb and borneol. According to the traditional Chinese medicine electuary, the selected medicinal materials achieve respective effects, administration is performed according to pathogenesis, the electuary has the effects of invigorating the spleen and supplementing qi, nourishing the liver and kidney, soothing the liver to relieve stagnation, clearing liver fire, calming liver wind, achieving tranquilization through heavy prescription, dissipating phlegm for resuscitation and tranquilizing mind by nourishing the heart, the absorption effect is good, the curative effect is remarkable, there is no toxic or side effect or adverse clinical reaction, and it is clinically verified that the cure rate of the tourette syndrome of children is high.
Owner:赵玉洁
Diagnostic assays to detect tumor antigens in cancer patients
PendingCN112424601APolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsOncology
The present invention generally relates to diagnostic assays using cell cultures, in particular to chimeric antigen receptor (CAR) expressing reporter cell assays to analyze samples, in particular patient samples, to diagnose cancer by quantifying the expression of tumor antigens and / or predicting clinical response to cancer immunotherapies. A further aspect of the present invention is to improvesafety of e.g., cancer immunotherapies.
Owner:F HOFFMANN LA ROCHE & CO AG
Recombinant herpesvirus turkey live vector vaccine capable of simultaneously expressing classical strain and variant infectious bursal disease virus VP2 protein
ActiveCN114107227AGuaranteed immune effectImprove securityViral antigen ingredientsVirus peptidesVector vaccineVariant strain
The invention discloses a recombinant herpesvirus turkey live vector vaccine capable of simultaneously expressing classical strain and variant strain infectious bursal disease virus VP2 protein, and belongs to the field of veterinary biological products. According to the invention, a VP2 protein and LTB fusion expression cassette containing an infectious bursal disease virus classical strain is knocked into a US2 virus replication non-essential region of a turkey herpesvirus, and a VP2 protein and LTB fusion expression cassette of a variant strain is inserted into a US10 virus replication non-essential region. The finally obtained recombinant virus simultaneously and efficiently expresses LTB-VP2 fusion antigen protein of the IBDV classic strain and the variant strain in CEF cells. The vaccine prepared by the invention can improve the antibody level after immunization, improve the uniformity of the antibody after immunization and ensure the immunization effect of the vaccine, has the advantages of high efficiency, good safety and lifelong immunization after one-time inoculation, does not cause clinical reaction and pathological damage to chicks, and has the vaccine protection rate of 100%.
Owner:扬州优邦生物药品有限公司
Compound kanamycin sulphate injection for dogs and preparation thereof
InactiveCN101297794BRelieve painReduce difficultyPharmaceutical delivery mechanismAntiviralsDoxofyllineAntibiotic Y
The invention discloses a compound kanamycin sulfate injection for dogs and a preparation method thereof, which aims at providing the compound kanamycin sulfate injection that has rapid onset of action for the treatment for canine parainfluenza and can ease the clinical reactions of the affected dogs, reduce the times and the dosage of the drug administration, alleviate the suffering of the dogs and the difficulty of the treatment operation, and the preparation method that has simple process and easy realization. Each 100l injection contains: 5 to 20 billion units of kanamycin monosulfate, 1 to 10kg of fosfomycin sodium, 0.1 to 2kg of doxofylline, 20kg of analgin, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na, 30kg of propylene glycol and the rest of water for injection. The injection of the invention is a compound preparation which combines antibiotics and drugs for the symptomatic treatment for usage, thus having rapid onset of action for the treatment for canine Para influenza and being able to ease the stress reactions of the affected dogs, prevent the secondary infection and accelerate the recovery of the dogs.
Owner:TIANJIN SHENGJI GRP CO LTD
Traditional Chinese medicine pill for laryngeal myasthenia ambulatory treatment and preparing method
InactiveCN105288275AGood curative effectHigh cure rateAnthropod material medical ingredientsMuscular disorderSide effectAmbulatory
The invention discloses a traditional Chinese medicine pill for laryngeal myasthenia ambulatory treatment and a preparing method, and belongs to the field of traditional Chinese medicine. The traditional Chinese medicine pill is prepared from, by weight, astragalus membranaceus, polished round-grained rice, radix ophiopogonis, rehmannia glutinosa, Chinese yams, radix scrophulariae, codonopsis pilosula, bighead atractylodes rhizomes, pericarpium citri reticulatae, angelica sinensis, common peony roots, red flowers, cimicifugae foetidae, radix bupleuri, terminalia chebula retz meat, schisandra chinensis, rhizoma acori graminei, oroxylum indicum, liquorice, peach seeds, lily, momordica grosvenori, scaphium scaphigerum, periostracum cicadas, bamboo juice and almonds. According to the traditional Chinese medicine pill, the selected medicinal materials conform to the monarch-and-minister matching principle, medicine is applied according to a disease cause, the traditional Chinese medicine pill has the effects of tonifying the lung and the spleen, tonifying the kidney and raising voices, and is good in absorbing effect, remarkable in curative effect and free of toxic and side effects and poor clinical reactions, and clinical tests show that the cure rate for laryngeal myasthenia is high.
Owner:孙娟
Method for prognosticating the clinical response of a patient to B-lymphocyte inhibiting or depleting therapy
ActiveUS8580528B2Reduce swellingSensitive to small effect of therapyMicrobiological testing/measurementAntibody ingredientsLymphocyteGene expression level
Owner:STICHTING VUMC
Big data-based adverse drug reaction rapid identification and disposal method and system
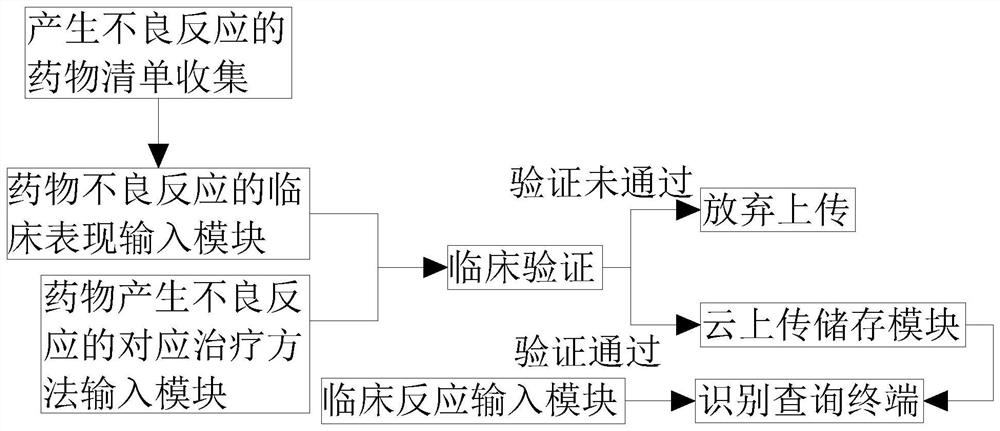
InactiveCN112820418ATimely treatmentFor easy referenceMedical practises/guidelinesDrug referencesClinical manifestationRapid identification
The invention provides a big data-based adverse drug reaction rapid identification and disposal method and system, and relates to the technical field of adverse drug reaction query. The big data-based adverse drug reaction rapid identification system comprises an adverse drug reaction list collection module, an adverse drug reaction clinical manifestation input module, an adverse drug reaction corresponding treatment method input module, a clinical verification module, a cloud uploading storage module, an identification query terminal and a clinical reaction input module. The identification query terminal is respectively connected with the clinical reaction input module and the cloud uploading storage module, and the cloud uploading storage module is in signal connection with a clinical verification module. By establishing an adverse drug reaction joint query system, adverse drug reactions found by all medical units are uploaded and summarized And the treatment scheme is verified, so that other medical units can conveniently refer to the treatment scheme, the adverse drug reaction can be treated in time, and the treatment rate of the adverse reaction is improved.
Owner:THE WEST CHINA SECOND UNIV HOSPITAL OF SICHUAN
Biomarkers for prostate cancer and methods for their detection
InactiveUS20170052185A1Good curative effectExcessive amountDisease diagnosisCancer antigen ingredientsProstate cancerEfficacy
The invention provides a method for predicting the clinical response to a cancer vaccine in a patient having cancer, a method for determining the immune response to a cancer vaccine in a patient having cancer who has been administered a cancer vaccine, a method for determining the long-term survival in a patient having cancer, corresponding kits therefor, as well as methods of for improving the efficacy of a virus-based vaccine.
Owner:UNITED STATES OF AMERICA
Biomarkers predictive for clinical response for glatiramer acetate
InactiveUS9617596B2Nervous disorderMicrobiological testing/measurementBiomarker (petroleum)Glatiramer acetate
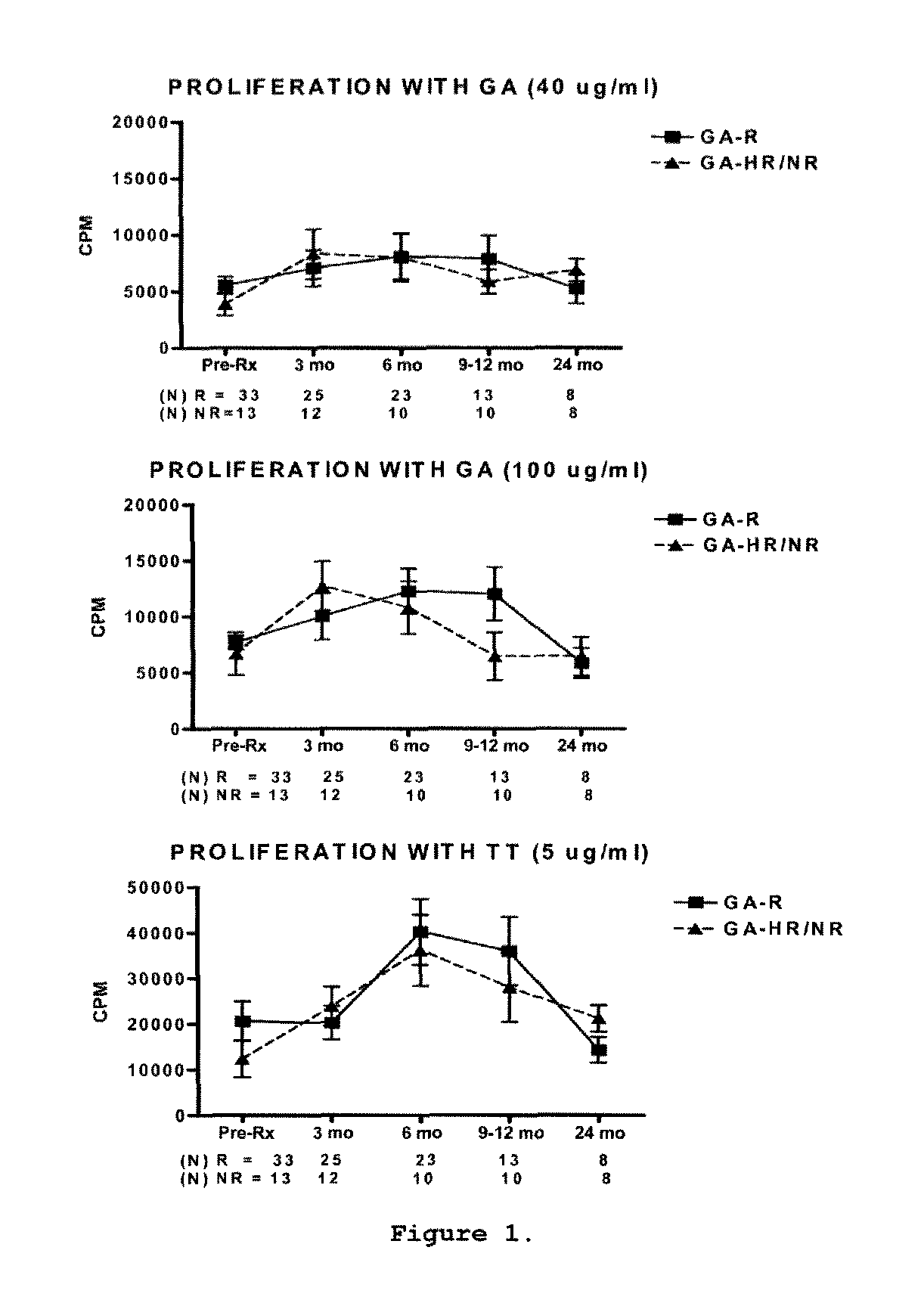
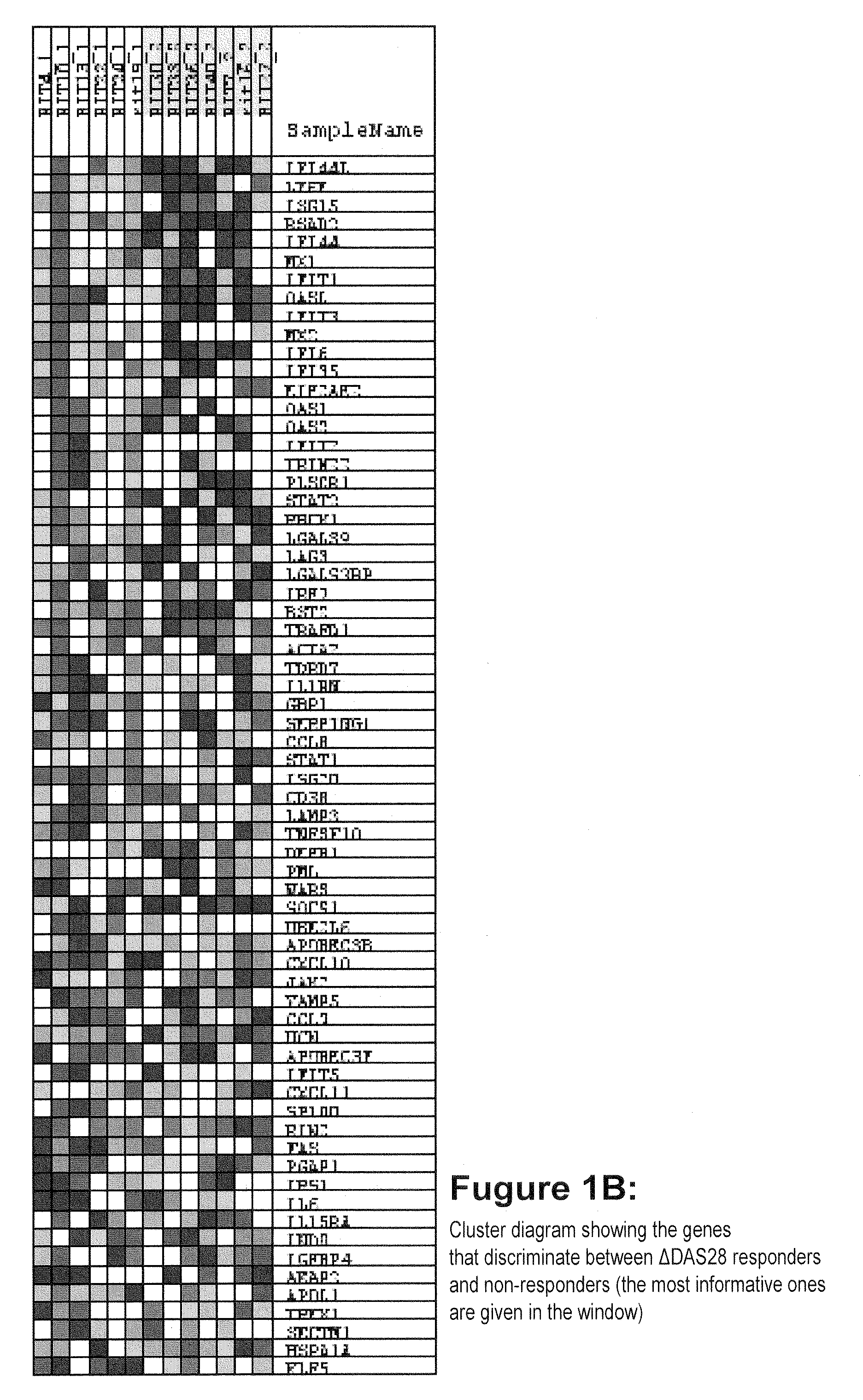
The present invention provides a method for treating a human subject afflicted with multiple sclerosis or a single clinical attack consistent with multiple sclerosis with a pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier, comprising the steps of:a) determining whether the human subject is a glatiramer acetate responder by evaluating expression of a biomarker selected from the group consisting of ERAP2, SIGLEC1, AAK1, KIAA1671, PLEKHA2, LOC730974, IFIT3, RWDD3, MYO6 SCARA3 and IFI44L, or a combination thereof, in human subject; andb) administering the pharmaceutical composition comprising glatiramer acetate and a pharmaceutically acceptable carrier to the human subject only if the human subject is identified as a glatiramer acetate responder.
Owner:TEVA PHARMA IND LTD
Pseudorabies virus mutant double gene deletion strain and its construction method and application
ActiveCN104059889BComplete immune protectionInactivation/attenuationMicroorganism based processesEnzyme digestionTransfer vector
The invention discloses a double gene-deleted strain of a pseudorabies virus variant, a construction method and an application thereof. The invention provides the pseudorabies virus strain with gI and gE genes being deleted. The pseudorabies virus variant is assigned the access number CGMCC. NO.8786. The invention further provides the construction method for the double gene-deleted strain. The construction method includes: (1) constructing a pseudorabies virus TJ strain transfer vector containing complete expression cassettes of EGFP and Neo; (2) transfecting the transfer vector to a cell which is inoculated with a pseudorabies virus TJ strain to obtain a transitional virus; and (3) performing an enzyme digestion process to a transitional viral genome, co-transfecting the enzyme digested transitional viral genome with the transfer vector and performing a plague screening process to obtain the double gene-deleted strain. The double gene-deleted strain is completely attenuated, is free of any clinical reaction after immunization of pigs, can rapidly induce generation of a specific antibody of pseudorabies virus, is high in neutralizing antibody titer and can provides a complete immune protection which aims at an attack of a presently-epidemic pseudorabies virus variant.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method for rhesus ovary and ovarian follicle generation model
ActiveCN102370973BFunction and effect are indeedAction and effect are stablePeptide/protein ingredientsSexual disorderMedicine.hematologyPhysiology
The invention provides a preparation method for a rhesus ovary and ovarian follicle generation model. According to the method, female rhesus monkeys are used as test objects, and each rhesus monkey is injected with 60 IU of luteinizing hormone LH each time and is injected twice everyday for seven days successively to prepare the rhesus ovary and ovarian follicle generation model. The invention also provides application of luteinizing hormone LH in preparing medicines for activating ovary and stimulating ovarian follicle thereof to grow. According to the invention, monkeys are used in the method for preparing the model, background data in physiology, hematology, blood biochemistry and the like of monkeys are abundant and easily available, monkeys have a biological background resembling to human beings, and therefore, test results of monkeys can well indicate clinical reactions of medicines; multi-aspect and multi-angle assessment are carried out, and obtained results can well and objectively indicate drug action on a reproductive system; animals (monkeys) and instruments (a B-mode ultrasonoscope, a microscope, a cell culture device, etc.) used in the model are simple and easily available, and the model has versatility.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +2
A kind of pill for treating chronic lymphocytic thyroiditis and its preparation method
InactiveCN104491364BHigh cure ratePromote absorptionInorganic active ingredientsDrug compositionsCitrus medicaAllium macrostemon
The invention discloses a pill for treating chronic lymphocytic thyroiditis and a preparation method, belonging to the field of traditional Chinese medicines. The effective components of the pill disclosed by the invention are composed of the following raw materials: 65-80 g of Chinese yam, 63-76 g of radix pseudostellariae, 60-73 g of immature bitter orange, 57-70 g of wolfberry, 55-67 g of semen litchi, 53-65 g of radix bupleuri, 50-62 g of white atractylodes rhizome, 43-55 g of the root of three-nerved spicebush, 40-52 g of glossy privet fruit, 38-50 g of stellaria aquatica, 35-47 g of tendril-leaved fritillary bulb, 32-45 g of pericarpium citri reticulatae viride, 30-42 g of fructus forsythiae, 28-40 g of rhizoma atractylodis, 25-37 g of liquorice, 23-35 g of allium macrostemon, 20-33 g of rose, 18-30 g of fingered citron, 15-28 g of selfheal, 12-25 g of sea horse, 9-22 g of oyster, 6-15 g of turtle shell, 4-10 g of pumex, and 2-7 g of air potato. The selected medicinal materials in the invention accord with monarch, ministerial and adjuvant principles; aiming at pathogenesis, treatment is carried out according to different symptoms; both symptoms and root causes are treated; the pill disclosed by the invention has the effects of tonifying the spleen and kidney, promoting blood circulation to remove blood stasis and resolving hard lump, and has the advantages of being good in absorption effect, obvious in curative effect and free from toxic and side effects and poor in clinical reaction; and in addition, found from clinical verifications, the pill disclosed by the invention has higher curative rate to chronic lymphocytic thyroiditis.
Owner:武威天健药业有限公司
Predictive test of Anti-tnf alpha response in patients with an inflammatory disease
InactiveUS20200165665A1Reduce severityImprove the situationMicrobiological testing/measurementWeissella cibariaSerratia fonticola
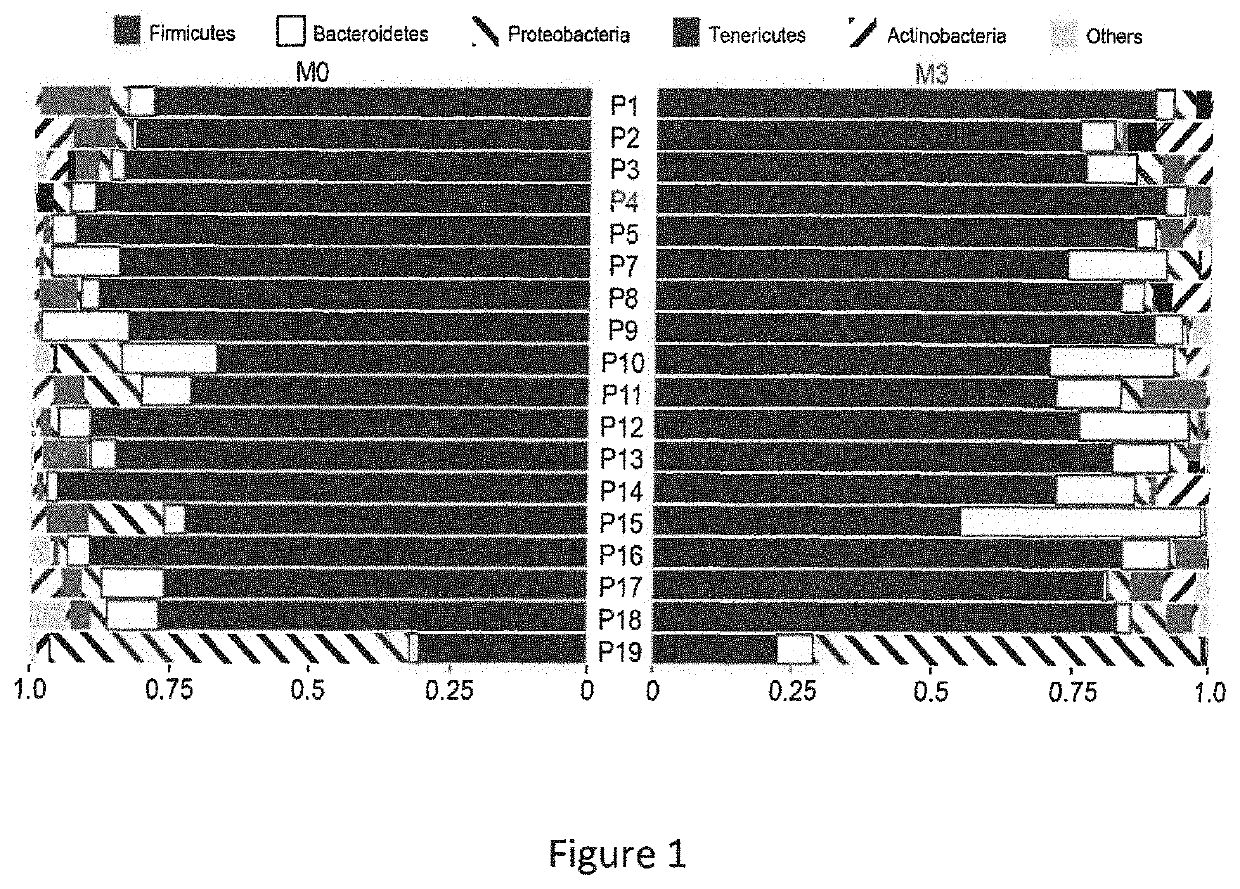
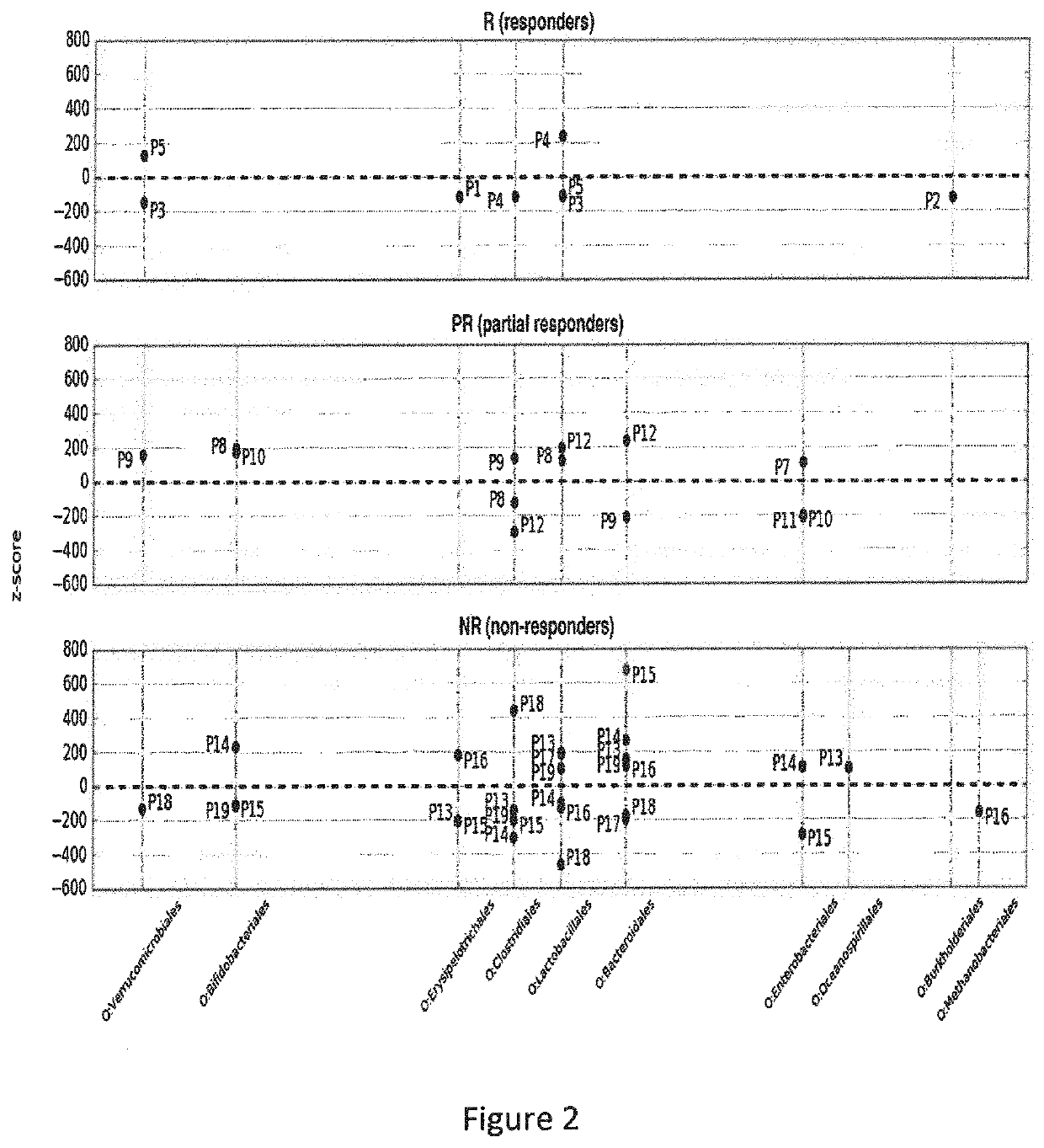
The present invention relates to an ex vivo method for predicting anti-TNF alpha response in a patient with an inflammatory disease in which this treatment is generally indicated, comprising the steps of: a) Measuring, before any anti-TNF alpha treatment, the level LM of Burkholderiales in a patient stool sample, and b) Calculating the score S1=LM / Lref, Wherein: ⋅If S1>1, the patient is considered likely to have a clinical response to an anti-TNF alpha treatment, or; ⋅If S1≤1, the patient is considered unlikely to have a clinical response to an anti-TNF alpha treatment, ⋅Lref being established on patients samples comprising a group (1) of patients with clinical improvement after treatment with TNF-alpha on the one hand, and a group (2) of patients who did not show any clinical improvement after treatment with TNF-alpha on the other hand, each of the groups (1) and (2) comprising at least 60 patients, by measuring the level of Burkholderiales at M0 in each of these groups, and determining the Lref value as the mean value separating patients from group (1) of patients in group (2). It also relates to an ex vivo method for predicting anti-TNF alpha response in a patient with an inflammatory disease in which this treatment is generally indicated, and to the use of at least one bacteria selected from the group comprising Burkholderiales, Serratia marcescens , Klebsiella oxytoca, Enterococcus gallinarum, Weissella cibaria and Coprococcus eutactus, as a predictive biomarker of the clinical outcome of an anti-TNF alpha treatment in an inflammatory disease.
Owner:UNIV DE BORDEAUX +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com