Big data-based adverse drug reaction rapid identification and disposal method and system
An adverse reaction and identification system technology, applied in drug reference, medical practical experience/guidance, electronic clinical trials, etc., can solve the problems of patients missing the best treatment and the inability to popularize adverse drug reactions, so as to avoid rescue time and facilitate treatment , the effect of improving the success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
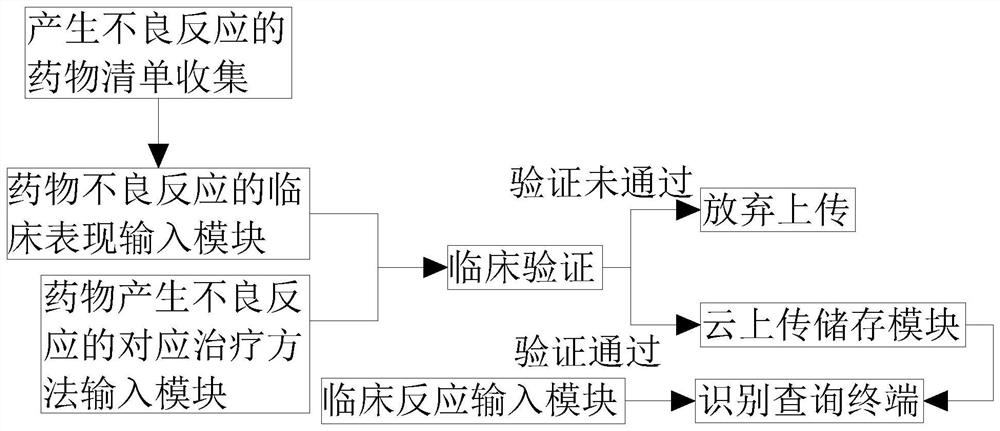
[0022] Such as figure 1 As shown, the embodiment of the present invention provides a rapid identification system for adverse drug reactions based on big data, including the collection of drug lists that cause adverse reactions, the clinical manifestation input module of adverse drug reactions, the corresponding treatment method input module for adverse drug reactions, A clinical verification module, a cloud upload storage module, an identification query terminal and a clinical response input module, the identification query terminal is respectively connected to a clinical response input module and a cloud upload storage module, and the cloud upload storage module is connected to a clinical verification module, so The above-mentioned clinical verification module is respectively connected with the clinical manifestation input module of the adverse drug reaction and the corresponding treatment method input module of the adverse drug reaction.
[0023] A method for rapid identific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com