A dual vaccine against avian metapneumovirus and h9 subtype avian influenza virus
A technology for avian metapneumovirus and avian influenza virus, applied in the direction of viruses, vaccines, antiviral agents, etc., can solve the problem of ineffective prevention of avian metapneumovirus and H9 subtype avian influenza, low antibody level and uniformity of antibodies, affecting vaccines Prevention and control effects and other issues, to achieve the effect of improving uniformity, increasing antibody levels, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Amplification and sequence analysis of F protein
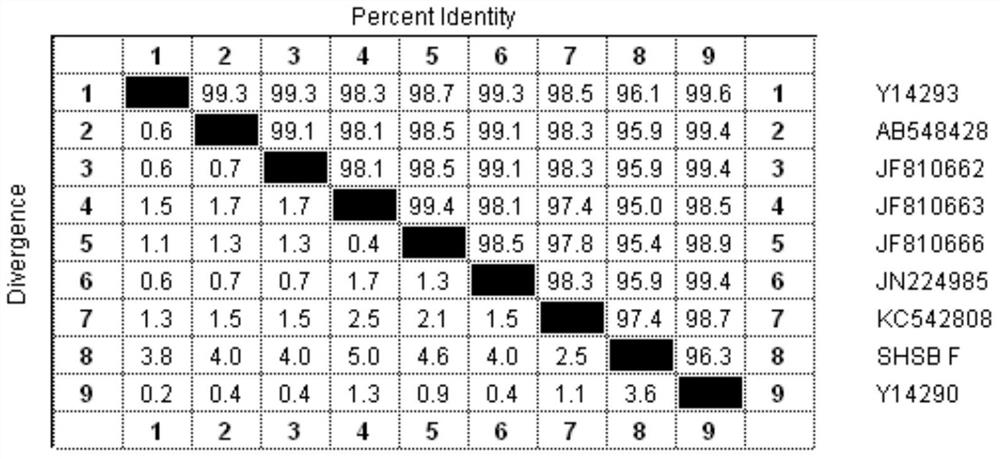
[0022] In 2010, symptoms of swollen head syndrome appeared in several breeding chicken farms in Shandong Province, and the affected chickens had been injected with the existing avian pneumovirus vaccine before, and it was speculated that the infected virus had mutated; Screening for lung viruses. Finally, an avian pneumovirus SHS / A3 was screened out.
[0023] In order to verify the antigenicity of the screened virus, virus strains from 5 different sources, including the screened strain SHS / A3, were used as antigens to prepare vaccines. After immunizing SPF chickens, the SHS / A3 strain virus solution was used to challenge the virus. The results It shows that compared with other poultry pneumovirus vaccines; the vaccine prepared by itself has a better immune effect (p<0.05); therefore, it is determined that genetic variation has occurred.
[0024] 1. Amplification of Type B Avian Metapneumovirus SHS / A3 Strain ...
Embodiment 2
[0035] Embodiment 2: Recombinant expression of F protein
[0036] 1. The preparation method of recombinant type B avian metapneumovirus F protein
[0037] The method comprises the following steps: a. constructing an expression vector; b. constructing an expression strain; c. inducing, extracting and purifying the recombinant F protein.
[0038] a. Construct expression vector:
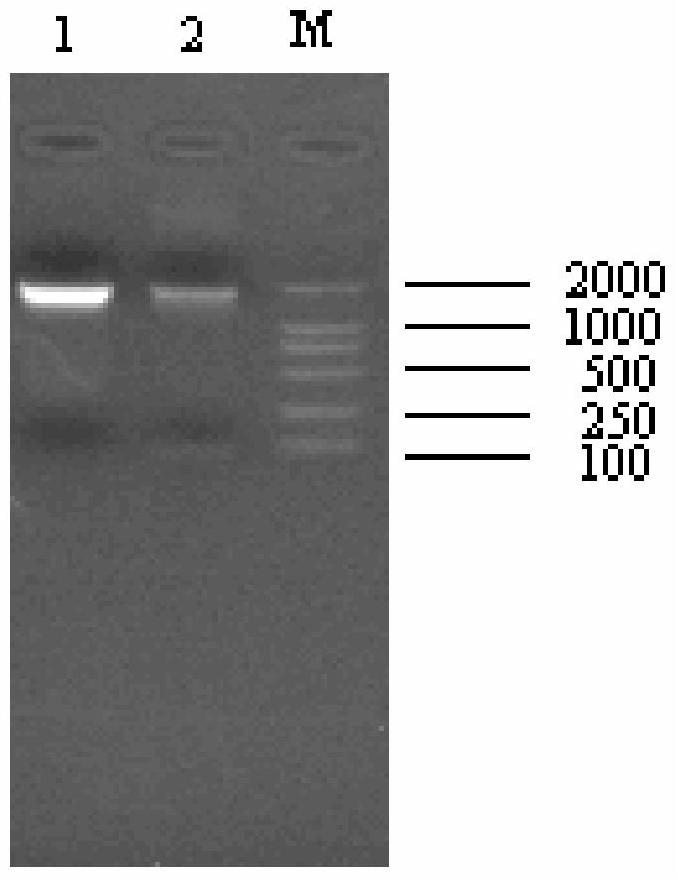
[0039] The positive clone plasmid pMD18-T-F and the expression vector pPICZα vector were double-digested with KpnI and NotI respectively, electrophoresed on a 1.2% agarose gel, and then recovered with a DNA gel recovery kit to obtain about 1.6kb and 3.3kb fragments, respectively. The pPICZα-F expression vector was constructed by directional ligation at 16°C ( Figure 4 It is the enzyme digestion identification diagram of the high-efficiency expression vector of the embodiment of the present invention); after sequencing and verifying that the sequence and reading frame are correct, the plasmid is linea...
Embodiment 3
[0044] Embodiment 3: Preparation of antigen for seedling production
[0045] 1. Preparation of avian influenza virus liquid for making seedlings Inoculate 10-11-day-old SPF chicken embryos through the allantoic cavity with the preservation number CCTCC NO:V201517 H9 subtype avian influenza virus QDY strain, 0.2ml per embryo, 36°C Incubate at ~37°C, take embryos twice a day, take dead chicken embryos after 24 hours, and harvest them at 96 hours, regardless of whether they are dead or not, cool at 4-8°C for 12-24 hours, harvest chicken embryo liquid, mix in sterile Store in a container at 2-8°C. The virus content of the harvested virus liquid is determined, and the virus content per 0.1ml is not less than 10 8.0 EID 50 ;
[0046]2. Inactivation Put the qualified venom into a sterilization container, add 10% formalin solution to a final concentration of 0.2%, and inactivate at 37°C for 18 hours;
[0047] 3. Preparation of F protein of avian metapneumovirus type B for seedling...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com