A kind of subunit vaccine for preventing type 4 avian adenovirus and its preparation method and application
A subunit vaccine and poultry adenovirus technology, applied in antiviral immunoglobulin, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of neutralizing immune deficiency, etc. degree, to ensure the effect of immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Construction of recombinant baculovirus
[0031] 1. Extraction of avian adenovirus genome: extract the total DNA of SD strain of avian adenovirus type 4 by traditional method;
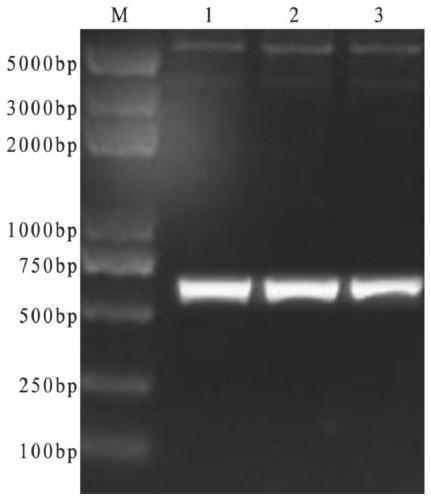
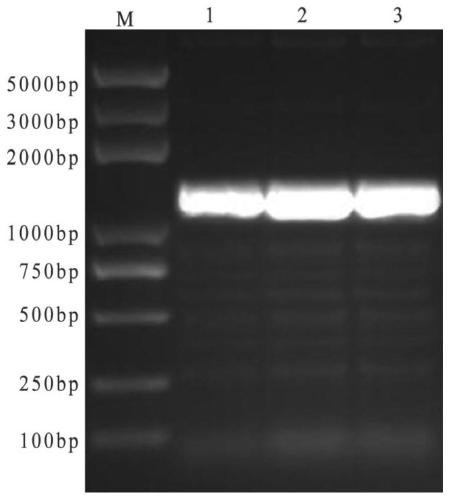
[0032] 2. Design and synthesis of primers: Using the self-isolated genome of avian adenovirus type 4 SD strain of group I as a template to analyze the antigenicity, hydrophilicity and surface display probability of the protein encoded by the FAVⅠhexon gene, select 494 amino acids at the N-terminus and 239 at the C-terminus One amino acid and two structural domains for baculovirus eukaryotic expression. A pair of specific primers were designed respectively according to the C-terminal and N-terminal domain gene sequences of the avian adenovirus Hexon gene published in GenBank, and Xho I and Sph I restriction sites were respectively added at both ends of the C-terminal primers, And the two ends of the N-terminal were added with BamH I and HindⅢ restriction sites respectively, and the...
Embodiment 2
[0049] Example 2: Preparation of C-terminal and N-terminal domain proteins of recombinant Hexon protein
[0050] 1. Recombinant baculovirus amplification: Inoculate the recombinant baculovirus rBacHexon-P10-C-PH-N into insect cells sf9, culture at 27°C for 4 days, collect the culture, centrifuge and take the supernatant to obtain the F2 generation recombinant rod virus;
[0051] 2. Identification of expressed protein:
[0052] (1) Insert the above-mentioned F2 generation recombinant baculovirus into insect cell sf9 with an inoculum amount of MOI=5-10, culture at 27°C and 110 rpm for 4 days, collect the culture, centrifuge and take the supernatant to obtain recombinant Hexon protein C -terminal and N-terminal domain proteins;
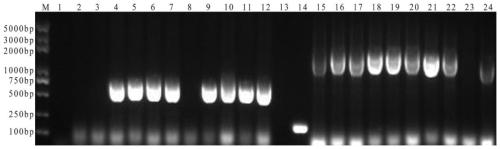
[0053] (2) SDS-PAGE identification: The above supernatant was subjected to SDS-PAGE electrophoresis; after electrophoresis, after staining and decolorization, it was found that the C-terminus was at about 27kDa and the N-terminus was at about 55kDa, an...
Embodiment 3
[0058] Embodiment 3: vaccine preparation
[0059] 1. Inactivation: Add the C-terminal and / or N-terminal domain protein of the recombinant Hexon protein prepared in large quantities in Example 2 into the inactivation tank, and add the final concentration of 0.2% to 0.5% inactivator BEI, Inactivate at 37°C for 24h.
[0060] 2. Inspection of semi-finished products
[0061] (1) Sterility test: carry out sterility test according to the appendix of the current "Chinese Veterinary Pharmacopoeia".
[0062] (2) Determination of protein content: the protein content was detected by Bradford method.
[0063] (3) Inactivation test: Insect cell sf9 was taken from the inactivated protein liquid, and cultured at 27° C. for 72 hours. No lesions were observed, and the inactivation test was judged to be qualified.
[0064] 3. Preparation of recombinant subunit vaccine:
[0065] The semi-finished protein antigen after passing the inspection is used for vaccine preparation (the liquid compone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com