Application of swine fever and porcine pseudorabies live vaccine to preparation of medicament for treating or preventing swine fever and porcine pseudorabies
A technology for swine pseudorabies and swine fever live vaccine is applied in the application field of swine fever and swine pseudorabies live vaccine, which can solve the problems of reducing vaccine immune protection, interfering with vaccine antibodies, reducing vaccine immune effect, etc., and preventing porcine respiratory syndrome. , Guarantee immune effect, break through the effect of interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 swine fever, porcine pseudorabies dual live vaccine
[0026] 1. Preparation of classical swine fever and porcine pseudorabies virus antigens
[0027] 1.1 Preparation of poisonous seeds for production
[0028] The preparation of the attenuated strain of classical swine fever rabbitization: the attenuated strain of classical swine fever rabbitization (purchased from the China Veterinary Drug Administration, the preservation number is AV1412) is properly diluted with the virus diluent (DMEM medium without serum), according to the multiplicity of infection ( M.O.I) was 0.1, inoculated on ST cell monolayer (purchased from CCTCC, No. GDC0060) and cultured, adsorbed at 37°C for 30 minutes, added DMEM cell maintenance solution containing 3% (v / v) calf serum, and cultured at 37°C for 3 to 5 days , freeze and thaw 2 to 3 times, harvest virus, virus titer ≥ 10 6.5 TCID 50 / ml.
[0029] Preparation of porcine pseudorabies virus gene deletion stra...
Embodiment 2
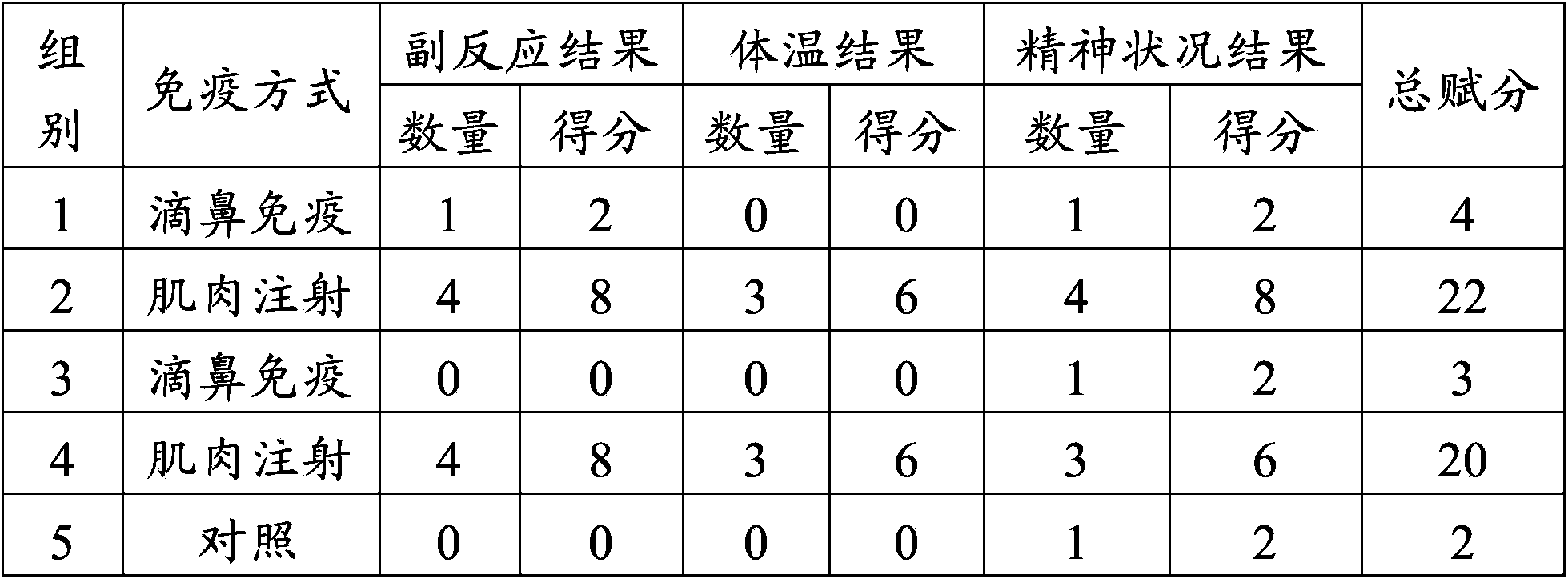
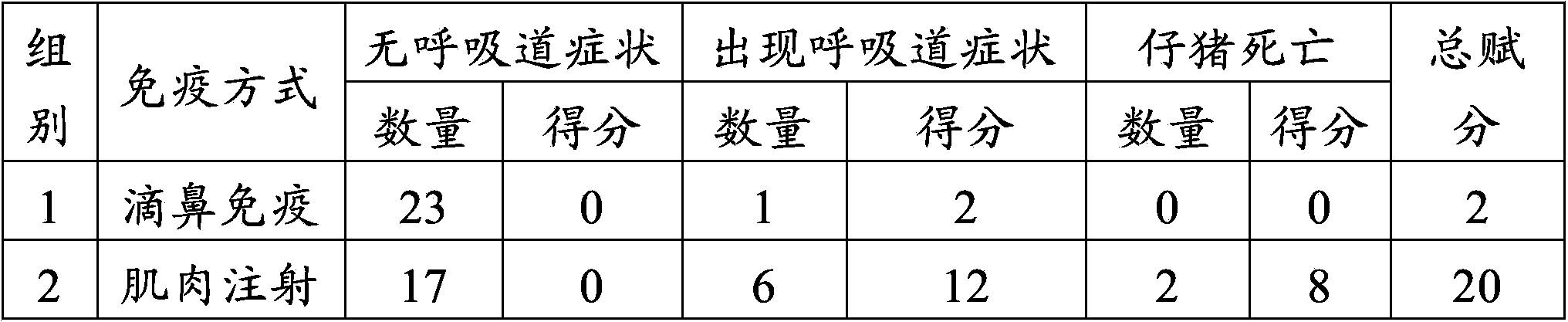
[0046] Example 2 The safety test of swine fever and porcine pseudorabies live vaccine in different immunization modes on piglets
[0047] 1. Materials
[0048] Live swine fever vaccine (rabbit attenuated strain) and porcine pseudorabies live vaccine (Bartha-K61 strain) were purchased from Pulaike Bioengineering Co., Ltd. (batch numbers 1207035 and 1202001B respectively). Vaccine (batch number is trial Z001) is prepared for embodiment 1.
[0049] 2. Design of animal experiments
[0050] Select 105 piglets at the age of 3 days after birth and divide them into 5 groups. Groups 1 to 4 are the test groups, 25 piglets per group. The piglets in the first group are inoculated with 2.0ml of live swine fever vaccine (2 piglets / head), the right side of the nasal cavity was inoculated with 2.0ml of live porcine pseudorabies vaccine (2 parts / head); the piglets of the second group were intramuscularly injected with 2.0ml of live swine fever vaccine (2 parts / head) in the left neck, and th...
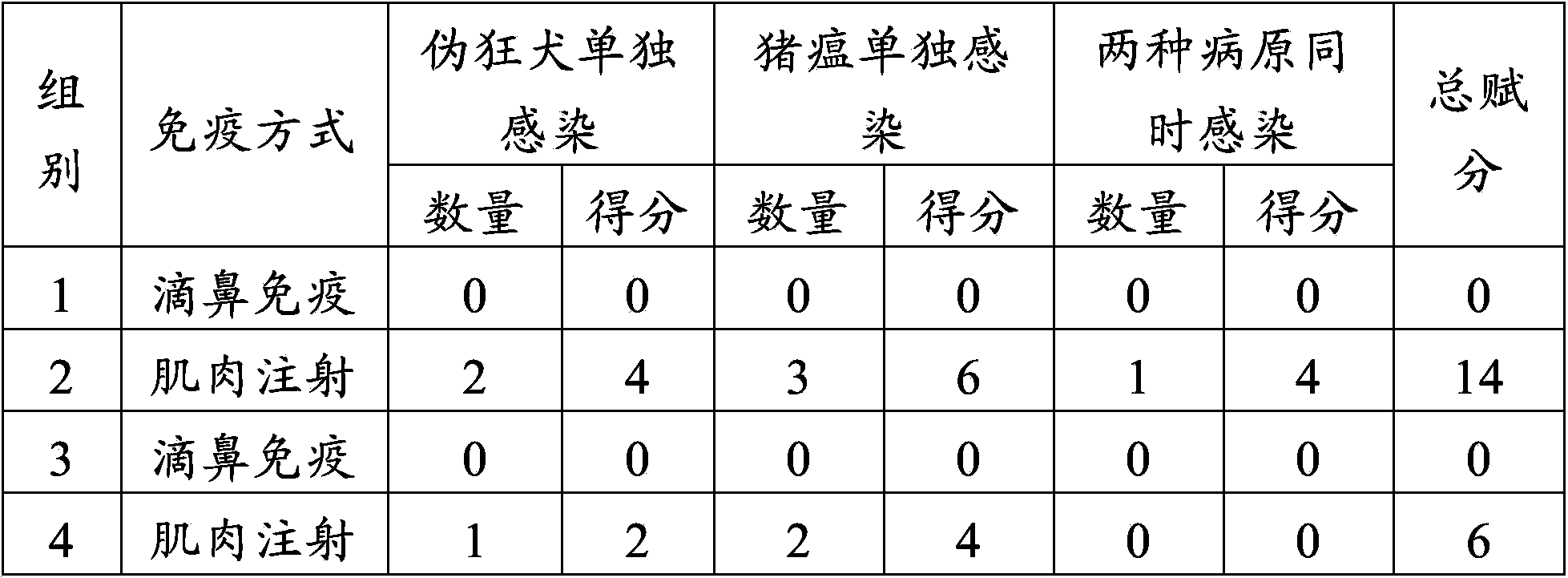
Embodiment 3
[0058] Example 3 Test of protective effect on piglets after early nasal drip immunization of swine fever and porcine pseudorabies live vaccine
[0059] 1. Materials
[0060] Live swine fever vaccine and porcine pseudorabies live vaccine were purchased from Pulaike Bioengineering Co., Ltd. (batch numbers 1207035 and 1202001B, respectively). The real-time fluorescent RT-PCR detection kit for wild strain of classical swine fever virus and the real-time fluorescent PCR detection kit for porcine pseudorabies virus (gE gene) were purchased from Beijing Century Yuanheng Animal Epidemic Prevention Technology Co., Ltd. The swine fever, porcine pseudorabies dual live vaccine (batch number is trial Z001) is prepared for embodiment 1.
[0061] 2. Design of animal experiments
[0062] Select 100 piglets at the age of 3 days after birth and divide them into 4 groups, 25 piglets per group. The piglets in the first group were inoculated with 1.0ml of live swine fever vaccine (1 dose / head) i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com