GPV (gosling plague virus) subunit vaccine as well as preparation method and application thereof
A technology of gosling plague virus and subunit vaccine, which is applied in the field of veterinary biological products, can solve the problems that no subunit vaccine of gosling plague has been successfully developed and applied, and is beneficial to the control and elimination of the disease, and the immunogenicity Good, low production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Construction of recombinant baculovirus
[0035] 1. Genome extraction of goose plague virus: extract the total DNA of goose plague virus TZ10 strain with TRIzol Reagent kit;
[0036] 2. Primer design and synthesis: Design a pair of specific primers based on the VP3 gene sequence of gosling plague virus published in GenBank, and add Sal I and Not I restriction sites at both ends of the primers to amplify the 1605bp target fragment. The primer sequences are:
[0037] P1: ACGCGTCGACATGGCAGAGGGAGGAG
[0038] P2: ATAAGAATGCGGCCGCTTACAGATTTTGAGTTAG
[0039] 3. Amplification and recovery of VP3 gene fragments
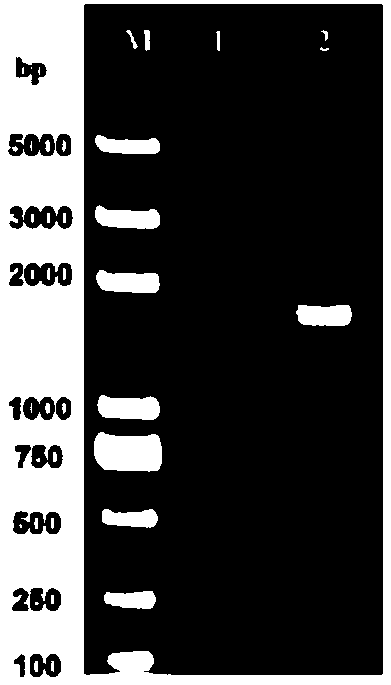
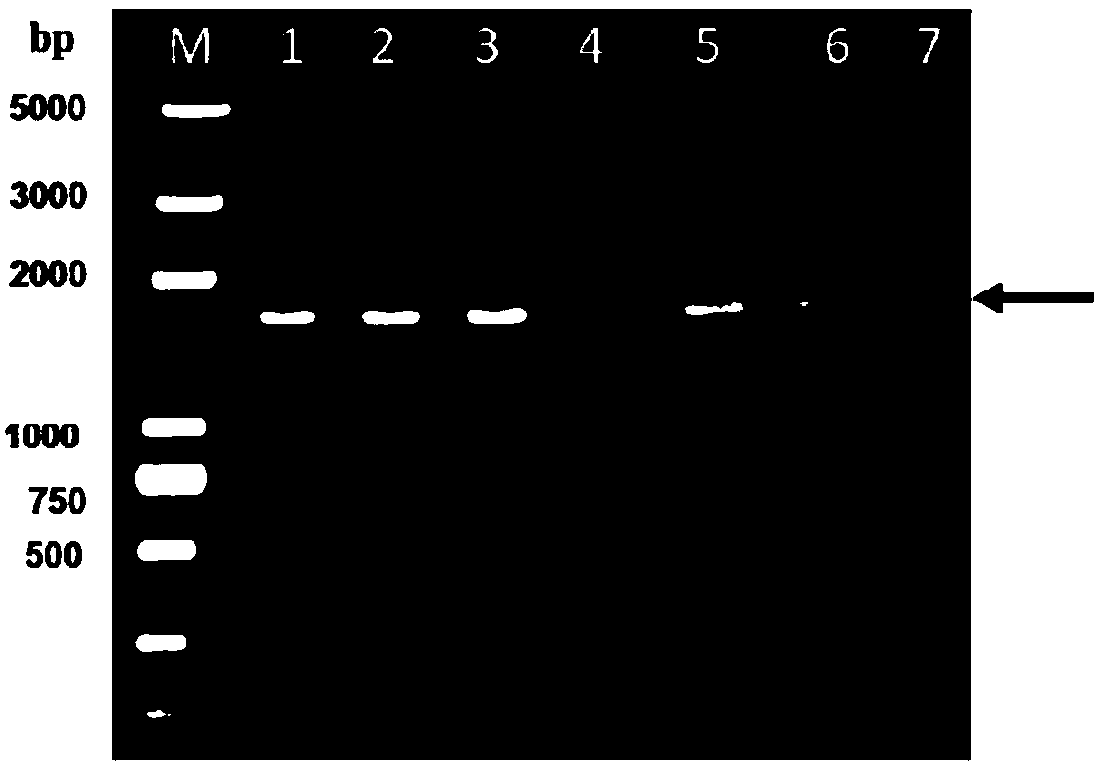
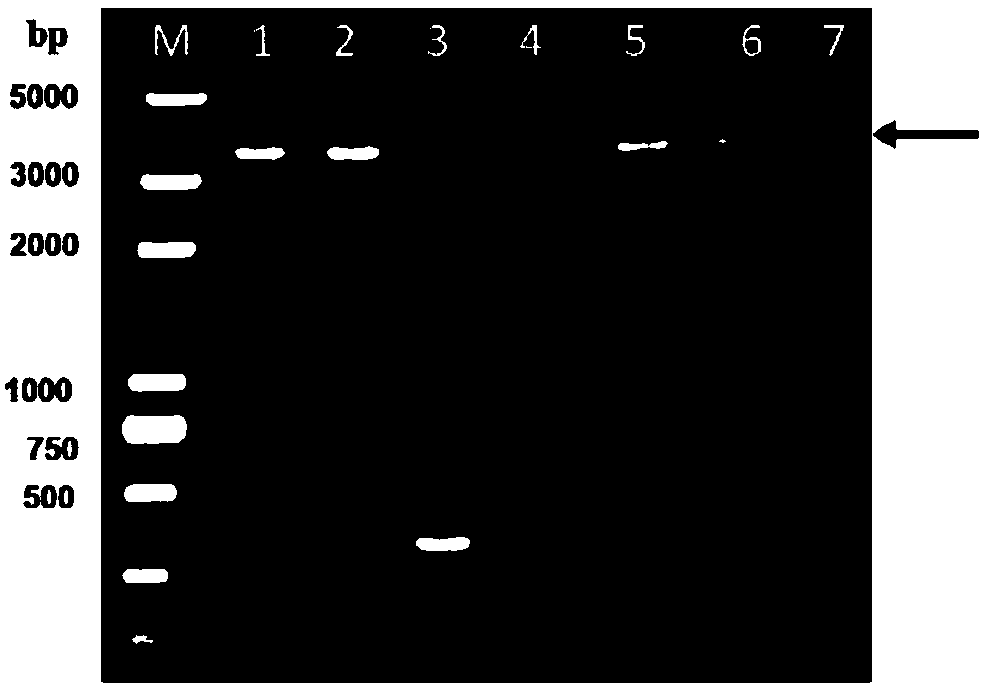
[0040] (1) PCR amplification: use the extracted DNA as a template, and perform PCR with the primers in step 2. The PCR reaction system is (total volume 25 μl): 0.5 μl of DNA template, 0.5 μl of P1 and P2, 12.5 μl of DNA polymerase and 11 μl of sterile water. The PCR reaction conditions are: 95°C, 5min; 30 cycles of 95°C for 30s, 65°C for 30s, 72°C fo...
Embodiment 2
[0048] 7. Recombinant bacmid transfection of Sf9 cells: transfect the purified recombinant bacmid into Sf9 cells by using liposome transfection method, the specific operation method refers to the Cellfectin of Thermo Fisher Scientific (China) Co., Ltd. According to the instructions of the transfection reagent, the F1 generation recombinant baculovirus GPVP3 / Bac strain was obtained. Embodiment 2: Preparation of recombinant VP3 protein
[0049] 1. Recombinant baculovirus amplification: Inoculate the recombinant baculovirus GPVP3 / Bac strain into insect cells Sf9, culture at 27°C for 4 days, collect the culture, centrifuge and take the supernatant to obtain the F2 generation recombinant baculovirus;
[0050] 2. Identification of expressed protein:
[0051] (1) Insert the above-mentioned F2 generation recombinant baculovirus into insect cell Sf9 at an inoculum amount of MOI=5-10, culture at 27°C for 4 days, collect the culture, centrifuge and take the supernatant to obtain the rec...
Embodiment 3
[0056] Embodiment 3: vaccine preparation
[0057] 1. Inactivation: Add the recombinant VP3 protein prepared in large quantities in Example 2 into an inactivation tank, add an inactivator BEI at a final concentration of 0.2% to 0.5%, and inactivate at 37° C. for 24 hours.
[0058] 2. Inspection of semi-finished products
[0059] (1) Sterility test: carry out sterility test according to the appendix of the current "Chinese Veterinary Pharmacopoeia".
[0060] (2) Determination of protein content: Serially dilute the BSA standard with known concentration, and perform SDS-PAGE together with the recombinant VP3 protein. After electrophoresis, use QuantityOne software to perform grayscale analysis, and compare the grayscale of the target protein and the standard protein To quantify the target protein. VP3 protein content is not less than 60μg / ml.
[0061] (3) Inactivation test: Insect cell Sf9 was taken from the inactivated protein liquid, and cultured at 27° C. for 72 hours. No ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com