Recombinant baculovirus and application thereof
A baculovirus and virus technology, applied in the field of gene therapy, can solve the problems of low virus yield, high production cost, unstable quality, etc., and achieve the effects of high virus quality, improved production capacity, and strong versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
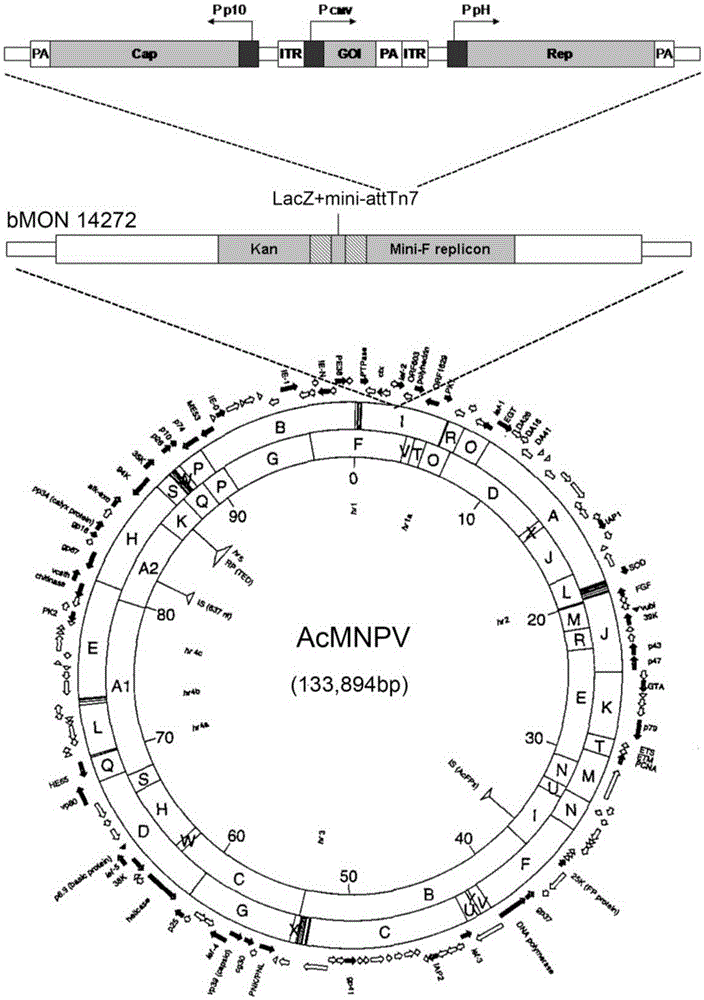
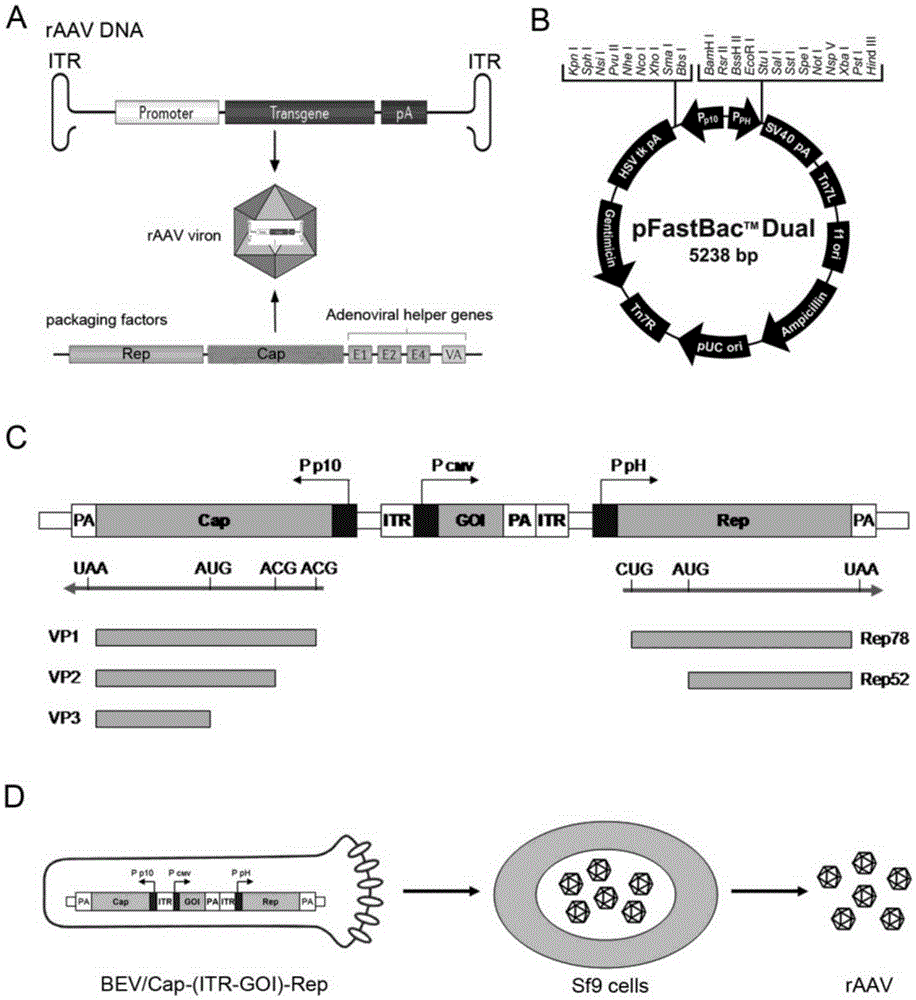
[0044] Embodiment 1: Preparation and amplification of recombinant baculovirus (BEV)
[0045] In order to put the three main components required for rAAV preparation: Cap gene, Rep gene and core expression element containing ITR into a recombinant baculovirus. We utilized the pFast.Bac.Dual (pFBD) shuttle vector from the baculovirus expression system Bac to Bac. In the example, the Rep gene based on type 2 AAV was codon-optimized according to the principle of ribosome leak scanning, and the Rep gene was placed under the regulation of the PpH promoter to realize the functional expression of Rep72 and Rep52 replication proteins. The Rep gene sequence is as shown in SEQ ID No.1, SEQ ID No.2 or SEQ ID No.3 (the three types correspond to RepA, RepB, and RepC). In the example, the Cap gene based on type 2 AAV was codon-optimized according to the principle of ribosome leak scanning, and the Cap gene was placed under the regulation of the P10 promoter to realize the three capsid pro...
Embodiment 2
[0052] Example 2: Utilize recombinant baculovirus (BEV) to infect Sf9 insect cells to prepare rAAV and verify its activity
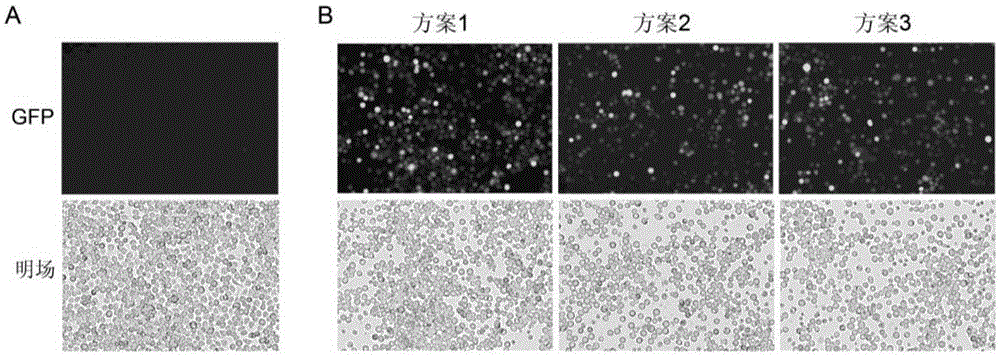
[0053] The BEV prepared in Example 1 was used to infect Sf9 cells in suspension culture at a multiplicity of infection (MOI=5). After 72 hours of infection, the cell culture solution was centrifuged at 3000 rpm for 5 min, and the culture supernatant and cell pellet were collected respectively. After BEV is produced, it is mainly released into the supernatant of the medium through secretion, and there are also some unreleased BEVs in Sf9 cells. After rAAV is produced, it mainly exists in the nucleus of Sf9 cells. Since Sf9 cells undergo cytopathic changes (CPE) after infection, some cells will be lysed, and rAAV will also be partially released into the supernatant. Therefore, both BEV and rAAV will be present in the supernatant and cell pellet.
[0054] In order to verify that Sf9 cells were infected with recombinant baculovirus, active rAAV was prepared...
Embodiment 3
[0056] Example 3: Purification and titer determination of rAAV
[0057] Because the rAAV prepared by the three combination schemes in Example 1 is practically the same, the rAAV prepared in Scheme 1 will be used as an example for the subsequent purification and activity detection of the rAAV prepared by the Bac-A system.
[0058] After the recombinant BEV was infected, about 1×10 8 Cells Sf9 cell pellet, add 10ml lysis buffer (50mM Tris-Cl, 150mM NaCl, 2mM MgCl 2 , pH 8.0), followed by repeated freezing and thawing 3 times, centrifuged at 5000rpm for 5min to collect the supernatant, added nuclease Benzonase to the supernatant to a final concentration of 50U / ml, and treated in a water bath at 37°C for 60min. After treatment, centrifuge at 5000rpm for 10min to collect the supernatant. The supernatant was extracted with chloroform, and the supernatant after the extraction was washed with 13.2% (NH 4 ) 2 SO 4 And the solution of 10% PEG8000 carries out the method for two-phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com