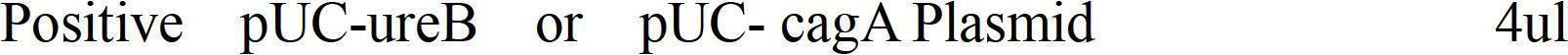Preparation process for gene vaccines containing UreB and CagA protein of helicobacter pylori
A Helicobacter pylori and gene vaccine technology, applied in the field of biomedicine, can solve the problem of low immune level and achieve the effect of solving low immune level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail through the following examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0023] 1 Construction and identification of fusion gene ureB-cagA:
[0024] Recombinant plasmids pUC-ureB and pUC-cagA were cut forward with EcoR Ⅰ and Pst Ⅰ respectively, the target fragments were recovered by tapping rubber, ligated and transformed, the resistant clones were picked and amplified, and the plasmids were extracted to obtain pUC-ureB-cagA. All identification.
[0025] 1.1 Digestion of recombinant plasmids
[0026]
[0027]
[0028] Digest for 1 hour at 37°C, stop the reaction with 2ul 10xLoadding Buffer, and electrophoresis on 1.0% agarose gel
[0029] 1.2 Construction and identification of recombinant plasmi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com