MAGE (Melanoma Antigen Gene)-4 anti-tumor CTL (Cytotoxic T Lymphocyte) epitope peptide and application thereof
A MAGE-4, 1.MAGE-4 technology, applied in the field of biochemistry, can solve the problem of not being able to achieve the effect of tumor suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of anti-tumor CTL epitope peptide P286 (Lys-Val-Leu-Glu-His-Val-Val-Arg-Val)
[0026] The Fmoc solid-phase synthesis method is used to extend one by one from the C-terminus to the N-terminus. Use fluorenylmethoxycarbonyl (Fmoc) to protect the α-amino group of amino acid, and the side chain protecting groups of various Fmoc protected amino acids are Arg (Pbf), His (trt), Glu (OtBu), Lys (Boc) (Pbf represents 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl, trt represents trityl, OtBu represents tert-butyl ester, Boc represents tert-butoxycarbonyl). First, use N, N'-diisopropylcarbodiimide (DIC) as the condensing agent for the first amino acid at the C-terminal of the α-amino protected C-terminal, and add 1-hydroxybenzotriazepam azole (HOBt), Fmoc-Val-OH is connected to the wang resin; sequentially washed with DMF, anhydrous methanol, dichloromethane, DMF; then piperidine-DMF mixture (V 哌啶 :V DMF =1:4) to remove the Fmoc protecting group, follo...
Embodiment 2
[0028] Example 2: Preparation of anti-tumor CTL epitope peptide P286-1Y2L (Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Val)
[0029] The same synthesis method as in Example 1 was adopted, except that the second N-terminal amino acid Val was replaced by Leu, and Lys was replaced by Tyr.
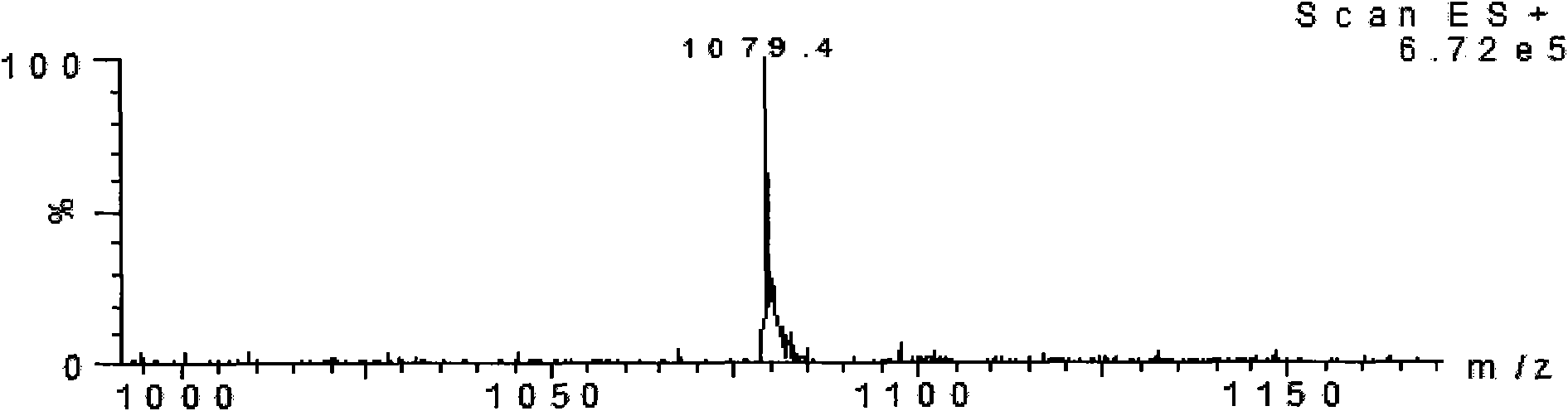
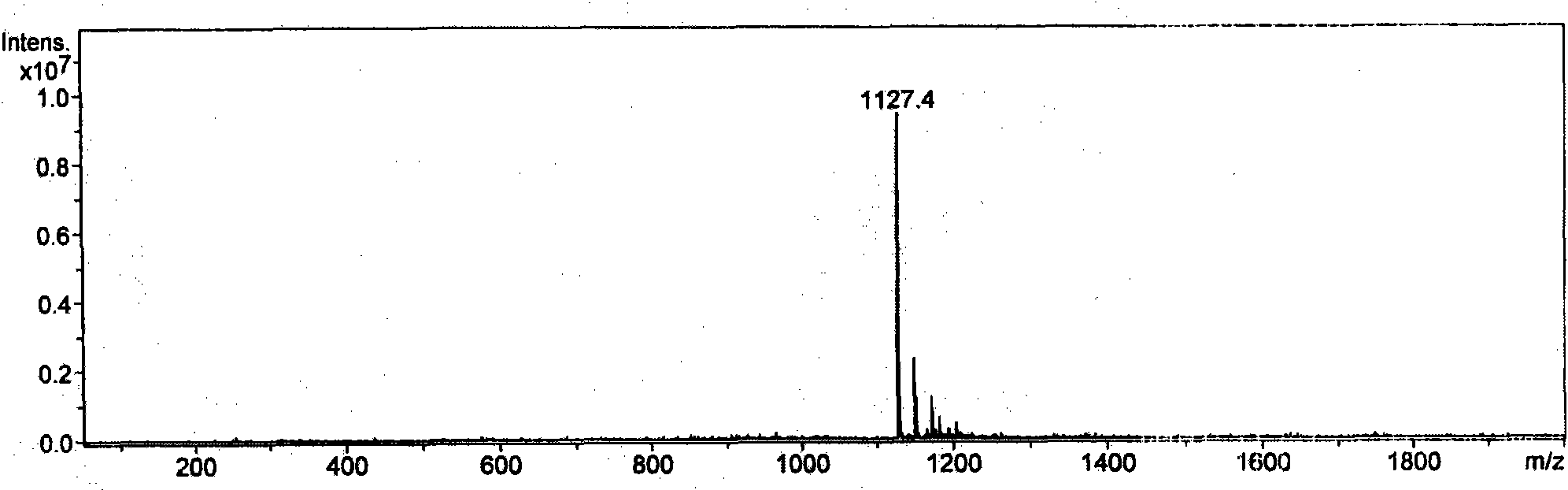
[0030] The product was analyzed by mass spectrometry, see figure 2 , the result confirmed that the molecular weight was 1127.4, which was consistent with the theoretical value.
Embodiment 3
[0031] Example 3: Preparation of anti-tumor CTL epitope peptide P286-1Y2L9L (Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Leu)
[0032] The same synthesis method as in Example 1 was adopted, except that the second amino acid Val at the N-terminus was replaced by Leu, the ninth amino acid Val at the N-terminus was replaced by Leu, and Lys was replaced by Tyr.
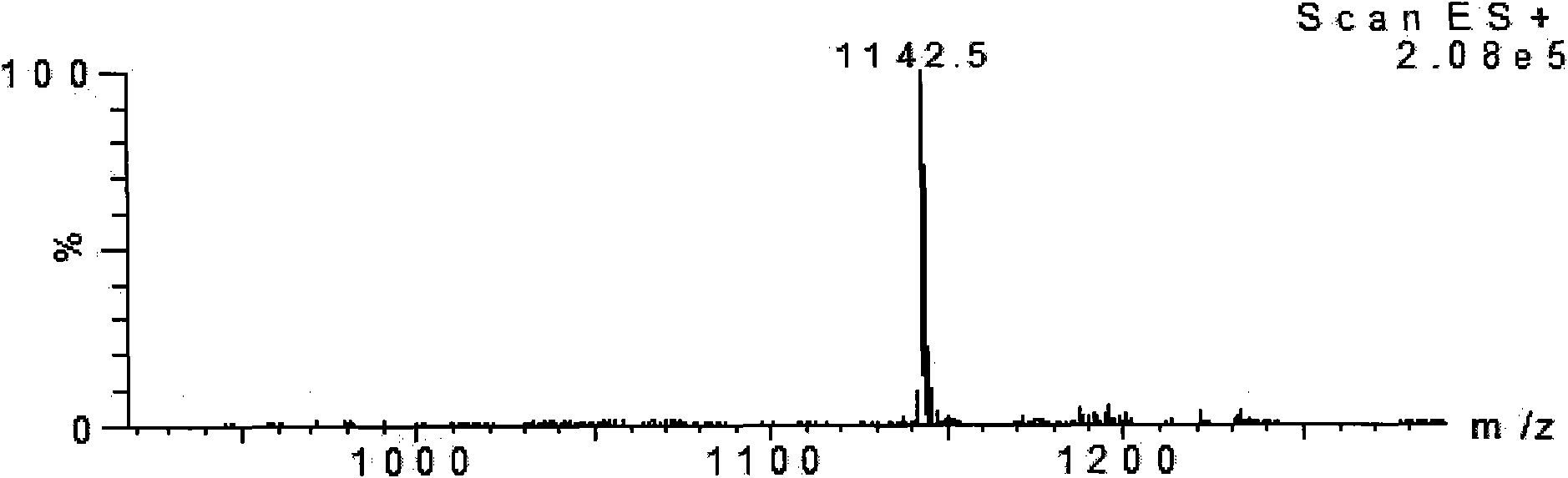
[0033] The product was analyzed by mass spectrometry, see image 3 , the results confirmed that the molecular weight was 1141.4, which was consistent with the theoretical value.
[0034] The CTL epitope peptide prepared above can be used to prepare a tumor therapeutic polypeptide vaccine, and its application experiment is as follows:
[0035] 1. Binding test of epitope peptide and HLA-A2.1 molecule
[0036] (1) Collect HLA-A2.1-positive T2 cells (ATCC company) with positive expression of HLA-A2.1 and lack of endogenous antigen processing ability by centrifugation at 800rpm, wash 3 times with phosphate buffered saline (PBS) at pH 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com