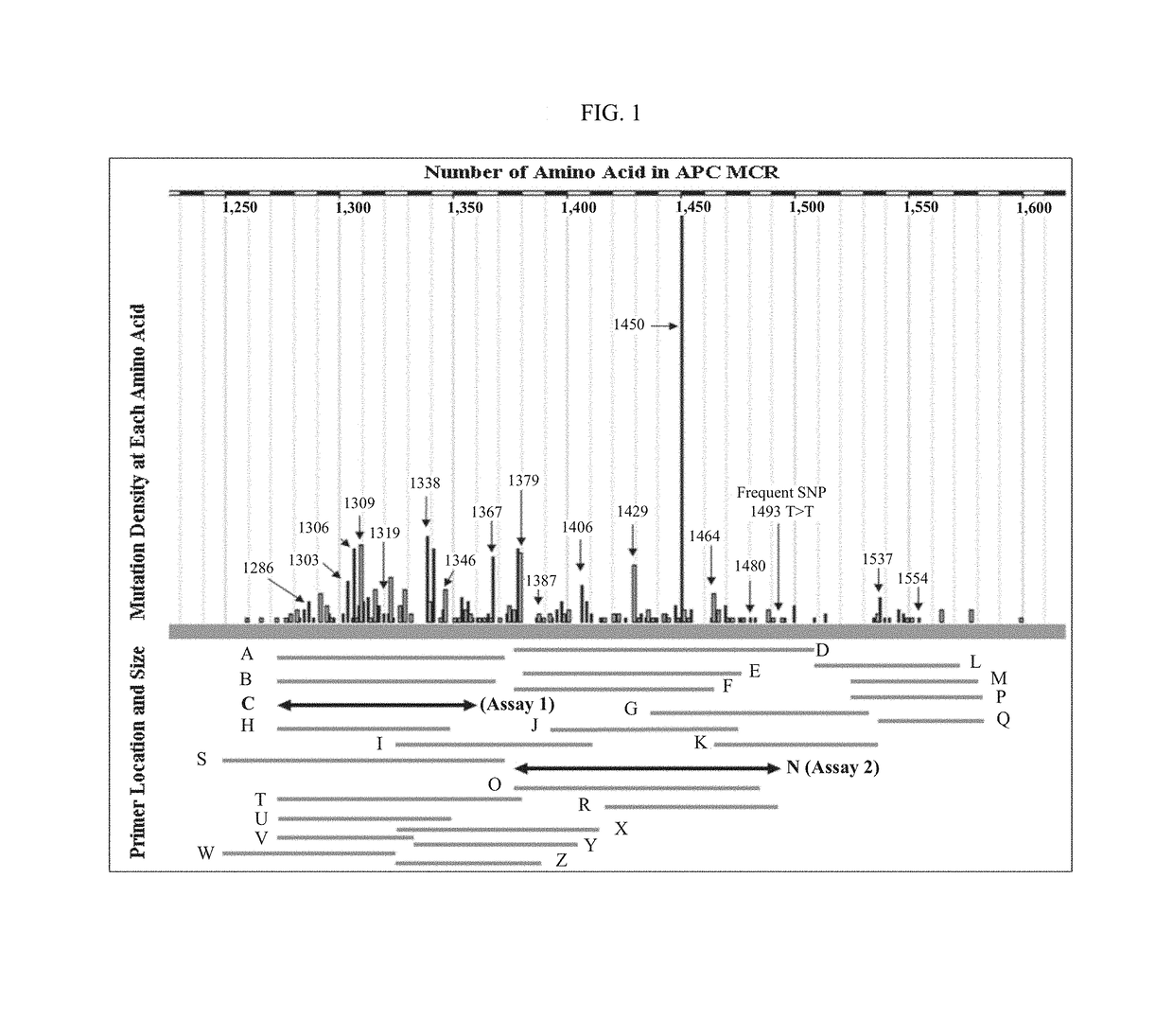
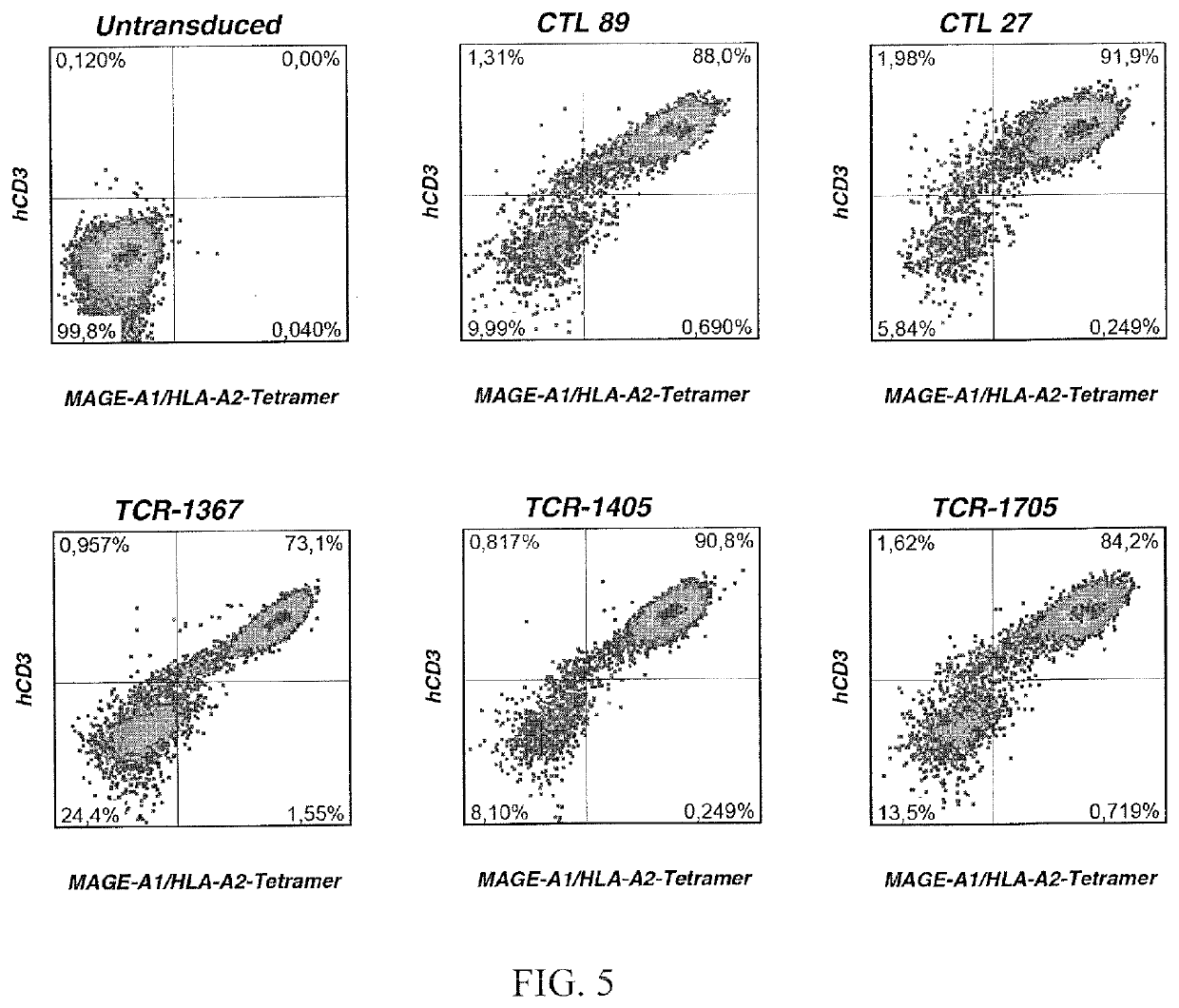
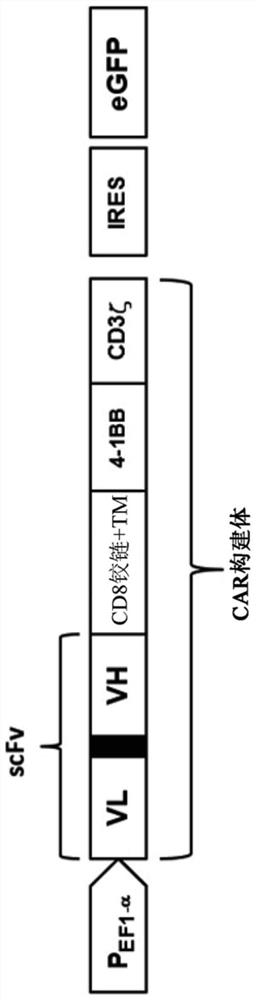
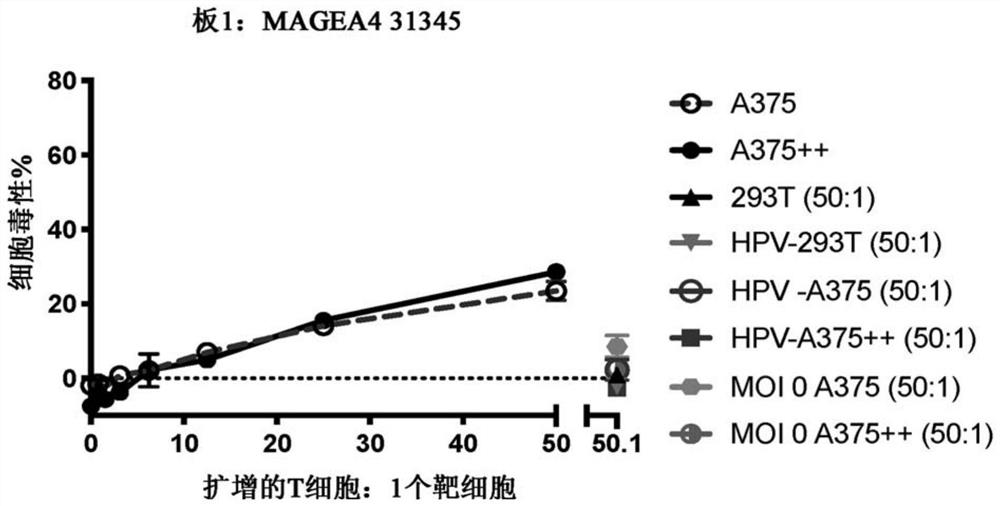
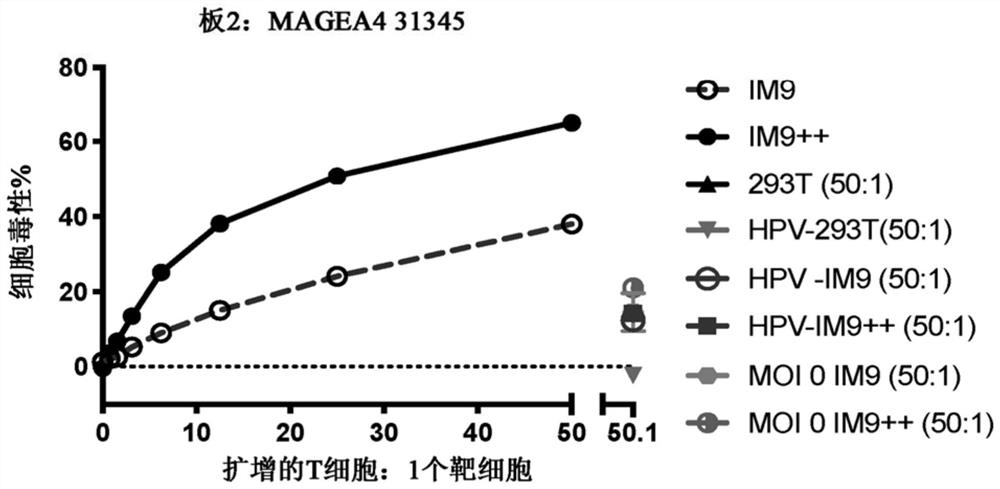
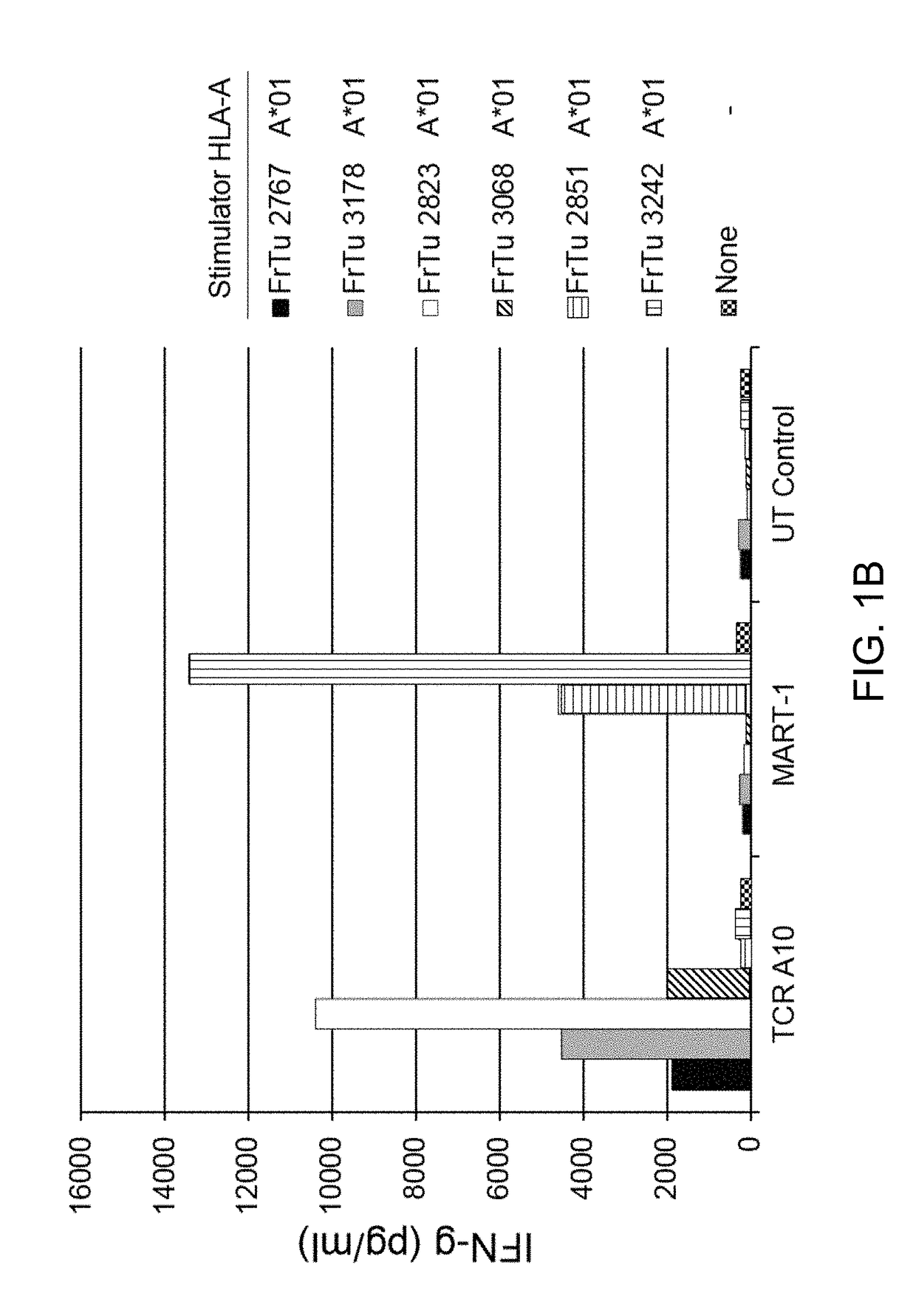
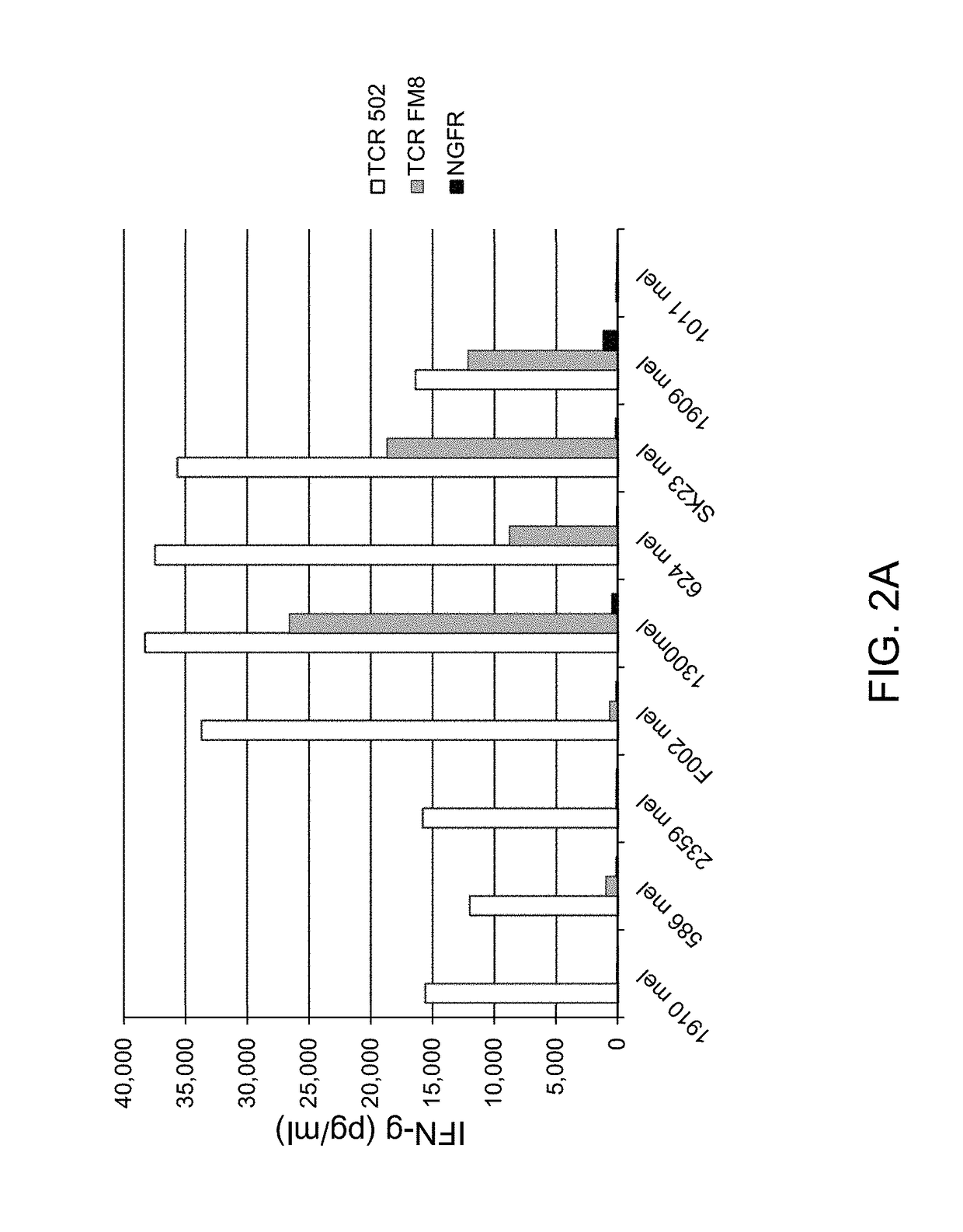
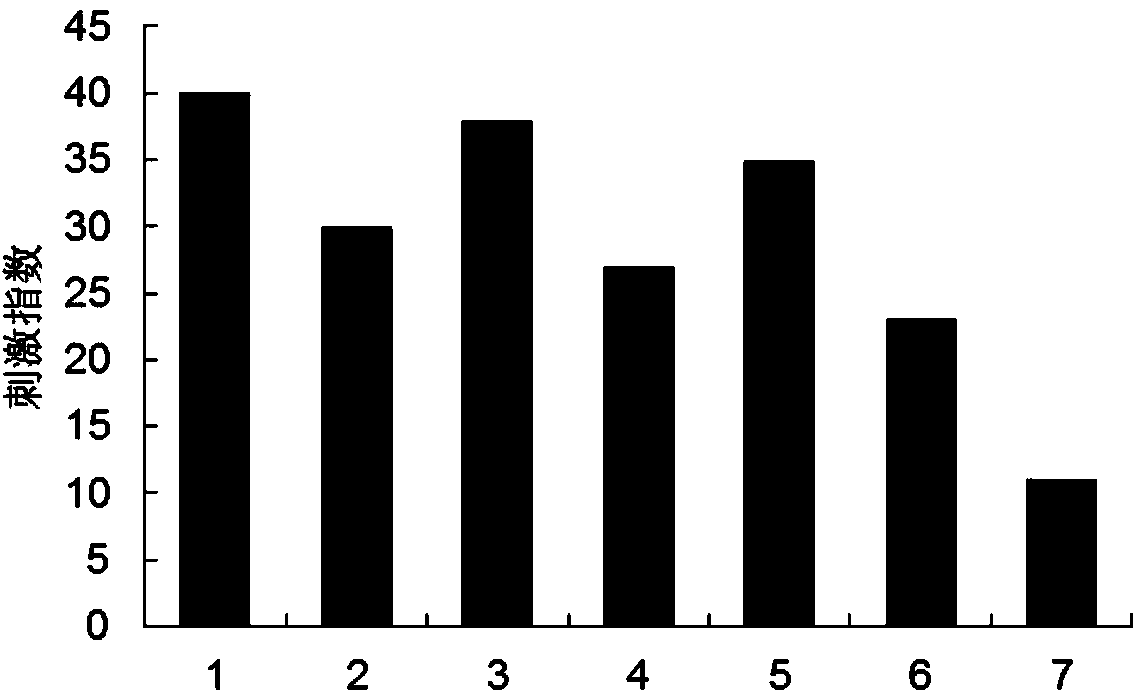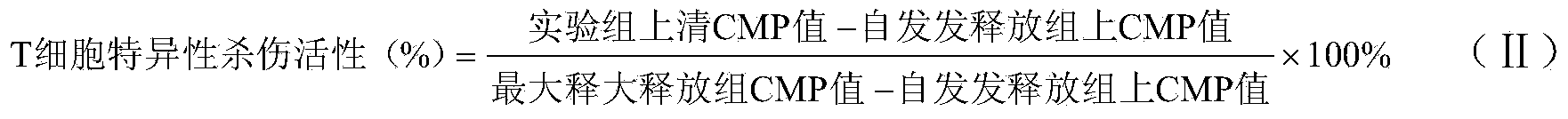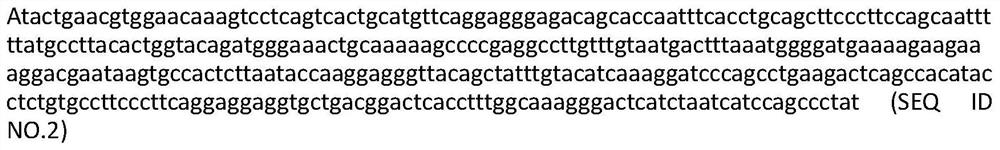Patents
Literature
37 results about "Melanoma Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The mammalian members of the MAGE (melanoma-associated antigen) gene family were originally described as completely silent in normal adult tissues, with the exception of male germ cells and, for some of them, placenta. By contrast, these genes were expressed in various kinds of tumors.
Composition and vaccine for treating lung cancer
InactiveUS20160168227A1High in proteinEffectively stimulating the (adaptive) immune systemOrganic active ingredientsTumor rejection antigen precursorsAntigenDisease
The present invention relates to a composition comprising at least one mRNA encoding a combination of antigens capable of eliciting an (adaptive) immune response in a mammal, wherein the antigens are selected from the group consisting of 5T4 (Trophoblast glycoprotein, TPBG), Survivin (Baculoviral TAP repeat-containing protein 5; BIRC5), NY-ESO-1 (New York esophageal squamous cell carcinoma 1, CTAG1B), MAGE-C1 (Melanoma antigen family C1), MAGE-C2 (Melanoma antigen family C2), and MUC1 (Mucin 1). The invention furthermore relates to a vaccine comprising at least one mRNA encoding such a combination of antigens, and to the use of said composition (for the preparation of a vaccine) and / or of the vaccine for eliciting an (adaptive) immune response for the treatment of lung cancer, preferably of non-small cell lung cancer (NSCLC), and diseases or disorders related thereto. Finally, the invention relates to kits, particularly to kits of parts, containing the composition and / or the vaccine.
Owner:CUREVAC AG
Isolated nucleic acid molecules which encode a melanoma specific antigen and uses thereof
InactiveUS6025191ATumor rejection antigen precursorsPeptide/protein ingredientsAntigenMelanoma-Specific Antigens
The invention involves the isolation of a nucleic acid molecule which encodes a melanoma associated antigen. Cell lines and expression vectors which include this and related sequences, as well as uses of these molecules, are described.
Owner:LUDWIG INST FOR CANCER RES LTD
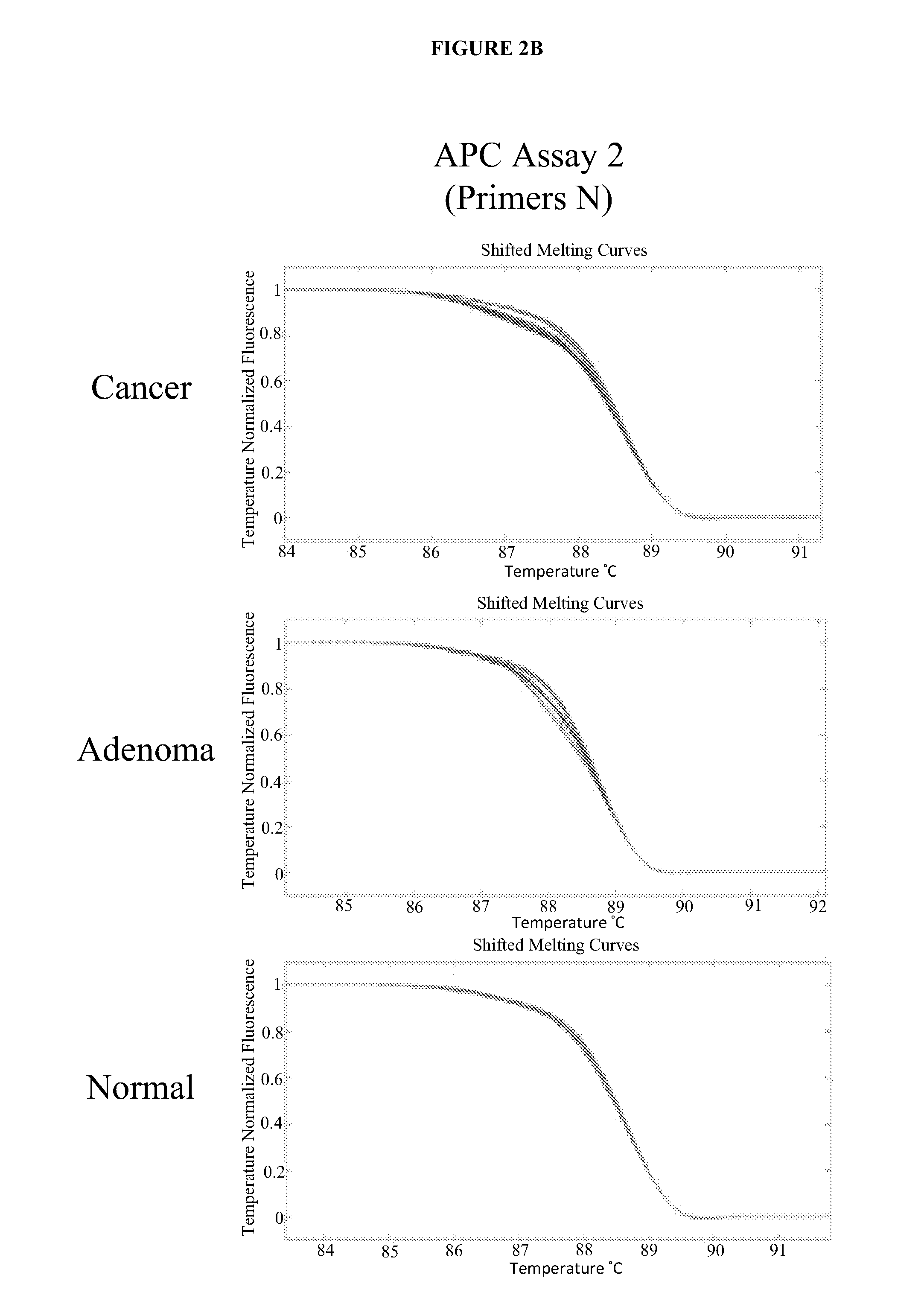
Methods and materials for detecting colorectal cancer and adenoma
InactiveUS20130012410A1High detectionHigh level of sensitivityMicrobiological testing/measurementLibrary screeningFOXE1Mammal
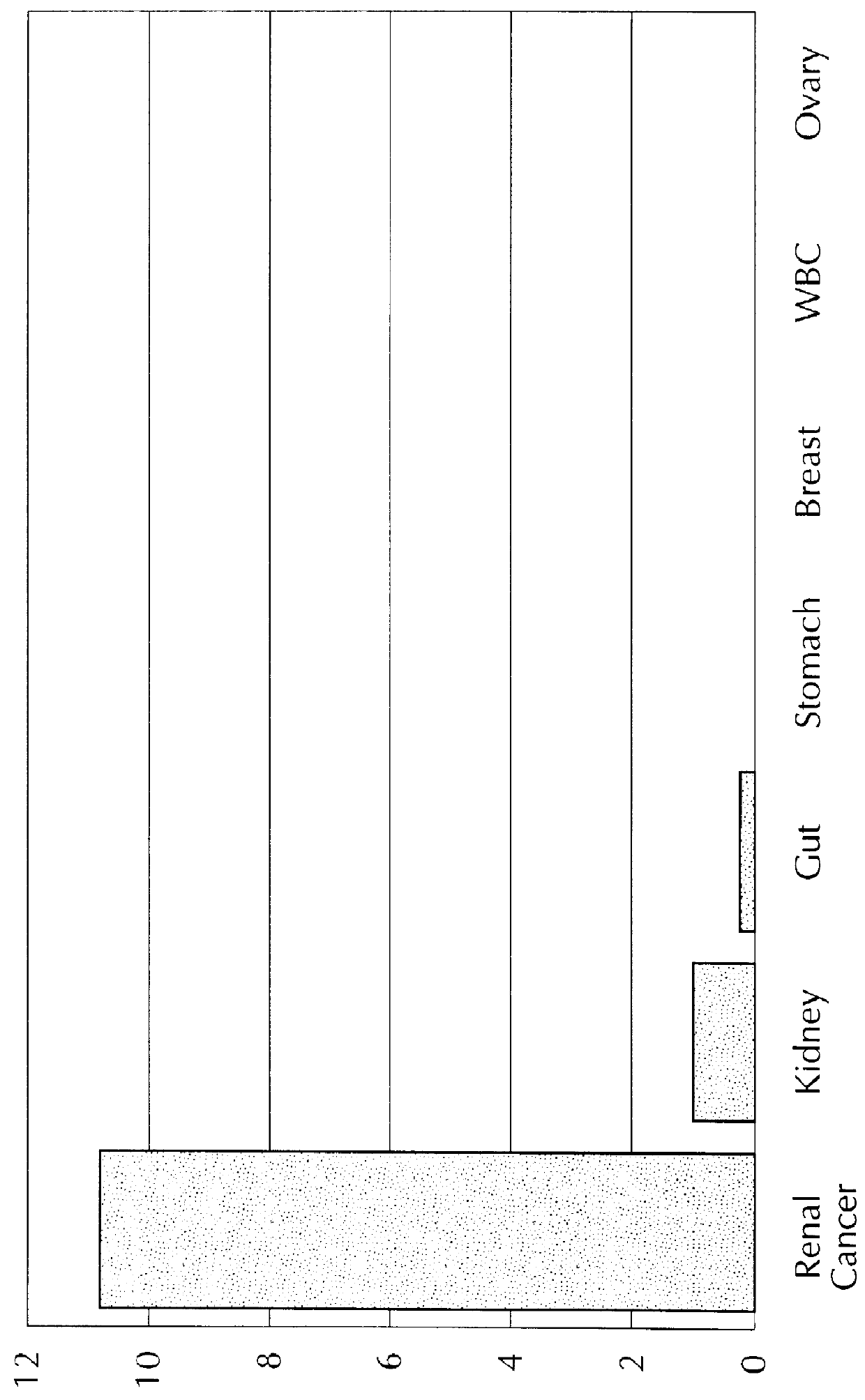
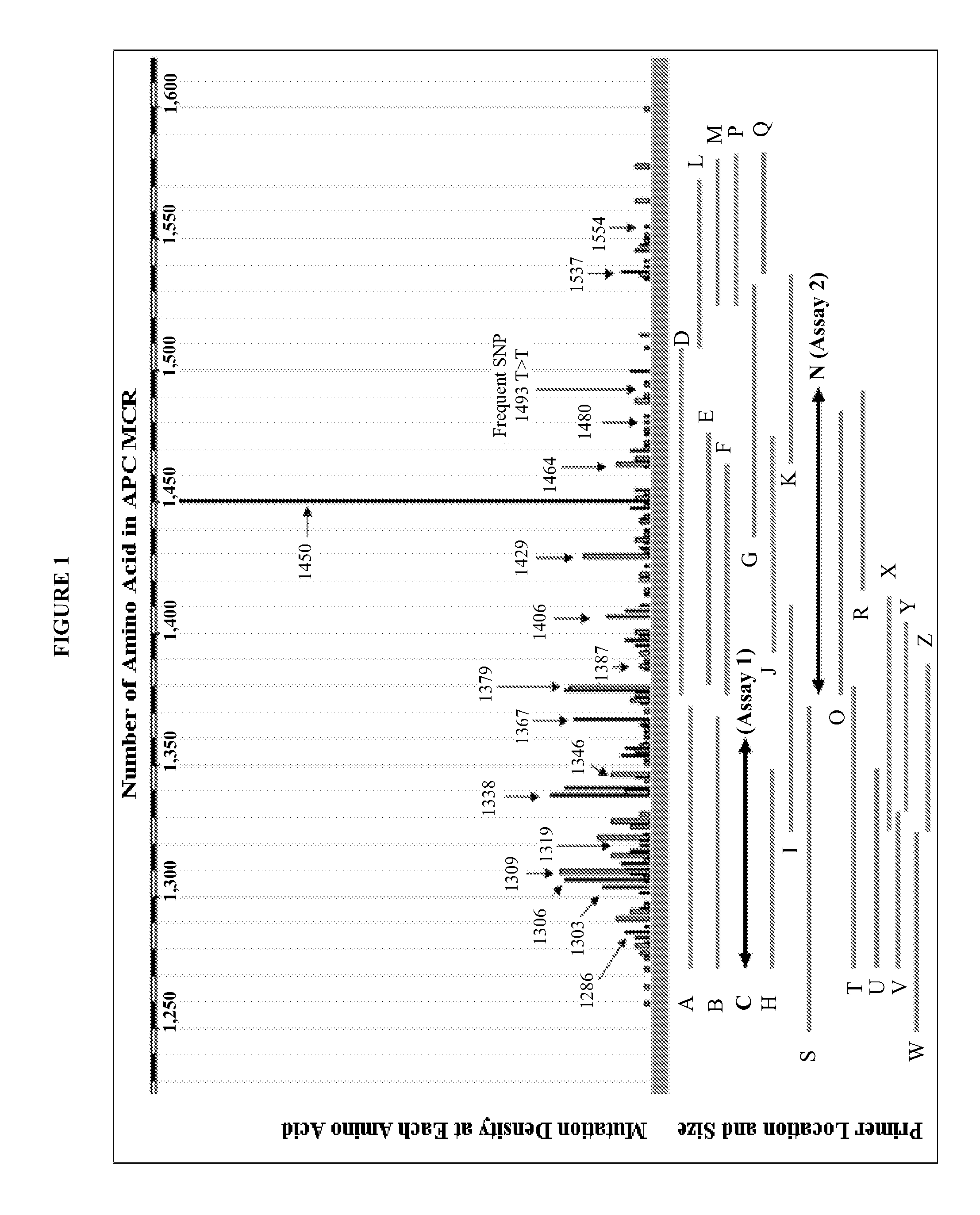
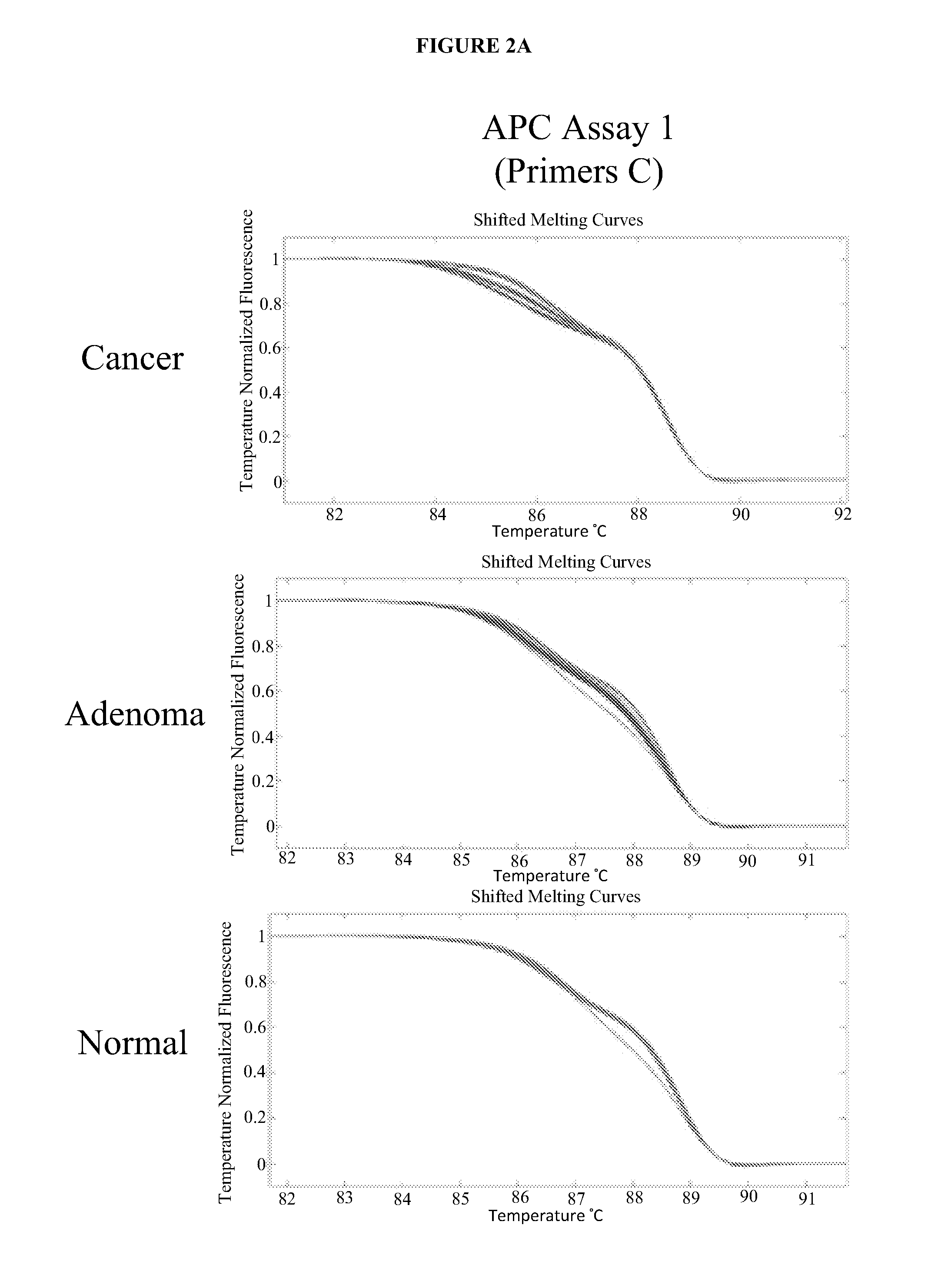
The present invention provides methods and materials related to the detection of colorectal neoplasm-specific markers (e.g., markers associated with colorectal cancer, markers associated with adenoma) in or associated with a subject's stool sample. In particular, the present invention provides methods and materials for identifying mammals (e.g., humans) having a colorectal neoplasm by detecting the presence and level of indicators of colorectal neoplasia such as, for example, long DNA (e.g., quantified by Alu PCR) and the presence and level of tumor-associated gene alterations (e.g., mutations in KRAS, APC, melanoma antigen gene, p53, BRAF, BAT26, PIK3CA) or epigenetic alterations (e.g., DNA methylation) (e.g., CpG methylation) (e.g., CpG methylation in coding or regulatory regions of bmp-3, bmp-4, SFRP2, vimentin, septin9, ALX4, EYA4, TFPI2, NDRG4, FOXE1) in DNA from a stool sample obtained from the mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
HLA binding peptides and their uses
InactiveUS20090012004A1Effective combinationSsRNA viruses positive-senseTumor rejection antigen precursorsImmunodeficiency virusBinding peptide
The present invention provides the means and methods for selecting immunogenic peptides and the immunogenic peptide compositions capable of specifically binding glycoproteins encoded by HLA alleles and inducing T cell activation in T cells restricted by the allele. The peptides are useful to elicit an immune response against a desired antigen. The immunogenic peptide compositions of the present invention comprise immunogenic peptides having an HLA binding motif, where the peptide is from a target antigen. Target antigens of the present invention include prostate specific antigen (PSA), hepatitis B core and surface antigens (HBVc, HBVs) hepatitis C antigens, Epstein-Barr virus antigens, melanoma antigens (e.g., MAGE-1), human immunodeficiency virus (HIV) antigens, human papilloma virus (HPV) antigens, Lassa virus, mycobacterium tuberculosis (MT), p53, CEA, trypanosome surface antigen (TSA) and Her2 / neu.
Owner:OSE PHARMA INT
T cell receptors recognizing hla-a1- or hla-cw7-restricted mage
InactiveUS20140378389A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen bindingRecombinant expression
The invention provides an isolated or purified T cell receptor (TCR) having antigenic specificity for a) melanoma antigen family A (MAGE A)-3 in the context of HLA-A1 or b) MAGE-A12 in the context of HLA-Cw7. The invention further provides related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells. Further provided by the invention are antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention. Methods of detecting the presence of cancer in a host and methods of treating or preventing cancer in a host are further provided by the invention.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
TCR (T Cell Receptor) for identifying MAGE-A1 (Melanoma antigen A1) oligopeptides
ActiveCN106749620AGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenOligopeptide
The invention provides a TCR (T Cell Receptor) which is capable of specifically binding oligopeptides KVLEYVIKV derived from an MAGE-A1 (Melanoma antigen A1). A compound can be formed by the oligopeptides KVLEYVIKV derived from the MAGE-A1 and an HLA (Human Leukocyte Antigen) A0201, and the oligopeptides KVLEYVIKV and the HLA A0201 can be transferred to cell surfaces together. The invention also provides a nucleic acid molecule for coding the TCR and a carrier comprising the nucleic acid molecule. In addition, the invention also provides a cell for transducing the TCR.
Owner:XLIFESC LTD
MAGE (Melanoma Antigen Gene)-4 anti-tumor CTL (Cytotoxic T Lymphocyte) epitope peptide and application thereof
ActiveCN101870725APeptide preparation methodsAntibody medical ingredientsCtl epitopeAbnormal tissue growth
The invention relates to an MAGE (Melanoma Antigen Gene)-4 anti-tumor CTL (Cytotoxic T Lymphocyte) epitope peptide. The epitope peptide is a nonapeptide, and the sequence of the nonapeptide is a-b-Leu-Glu-His-Val-Val-Arg-c, wherein a is Lys or Tyr, b is Val or Leu, and c is Val or Leu; the sequence of an epitope peptide P286 is Lys-Val-Leu-Glu-His-Val-Val-Arg-Val; the sequence of an anti-tumor CTL epitope peptide P286-1Y2L is Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Val; and the sequence of an anti-tumor CTL epitope peptide P286-1Y2L9L is Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Leu. The specific CTL induced in the body of a transgenic mouse by the anti-tumor CTL epitope peptide has certain kill rate to tumor cells EC-9706; and the epitope peptide can be used for preparing therapeutic tumor polypeptide vaccines.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Inhibition of breast carcinoma stem cell growth and metastasis
InactiveUS20070297983A1Growth inhibitionIn-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenLymphatic Spread
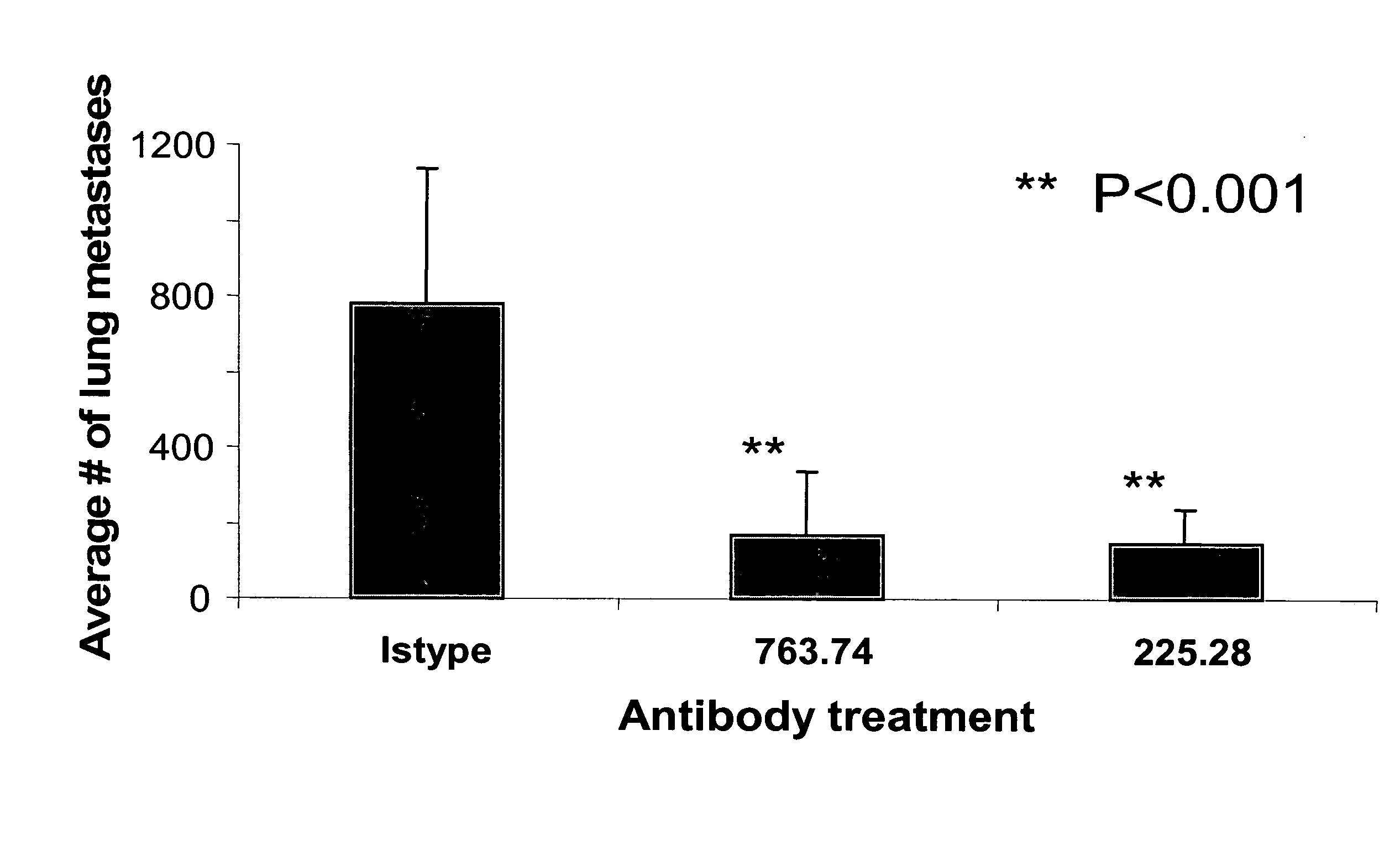
Disclosed is a method for inhibiting the growth of breast carcinoma stem cells. that express High Molecular Weight -Melanoma Associated Antigen (HMW-MAA). The method comprises administering to an individual a composition comprising an antibody reactive with HMW-MAA or a fragment of such an antibody in an amount effective to inhibit the growth of the breast carcinoma cells. Also provided are methods for inhibiting metastasis of breast carcinomas and methods for identifying HMW-MAA+ breast cancer stem cells.
Owner:DUKE UNIV +1
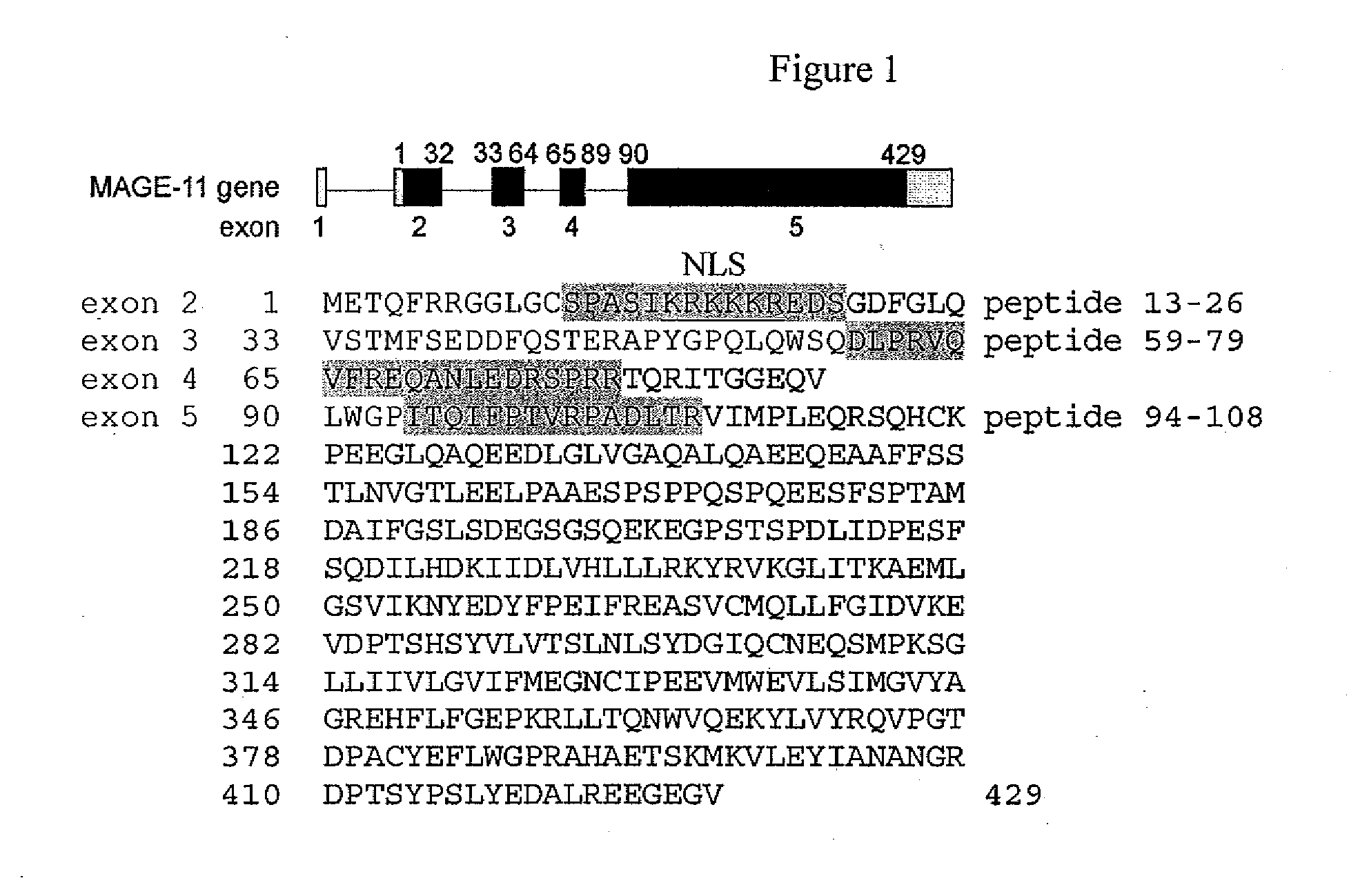
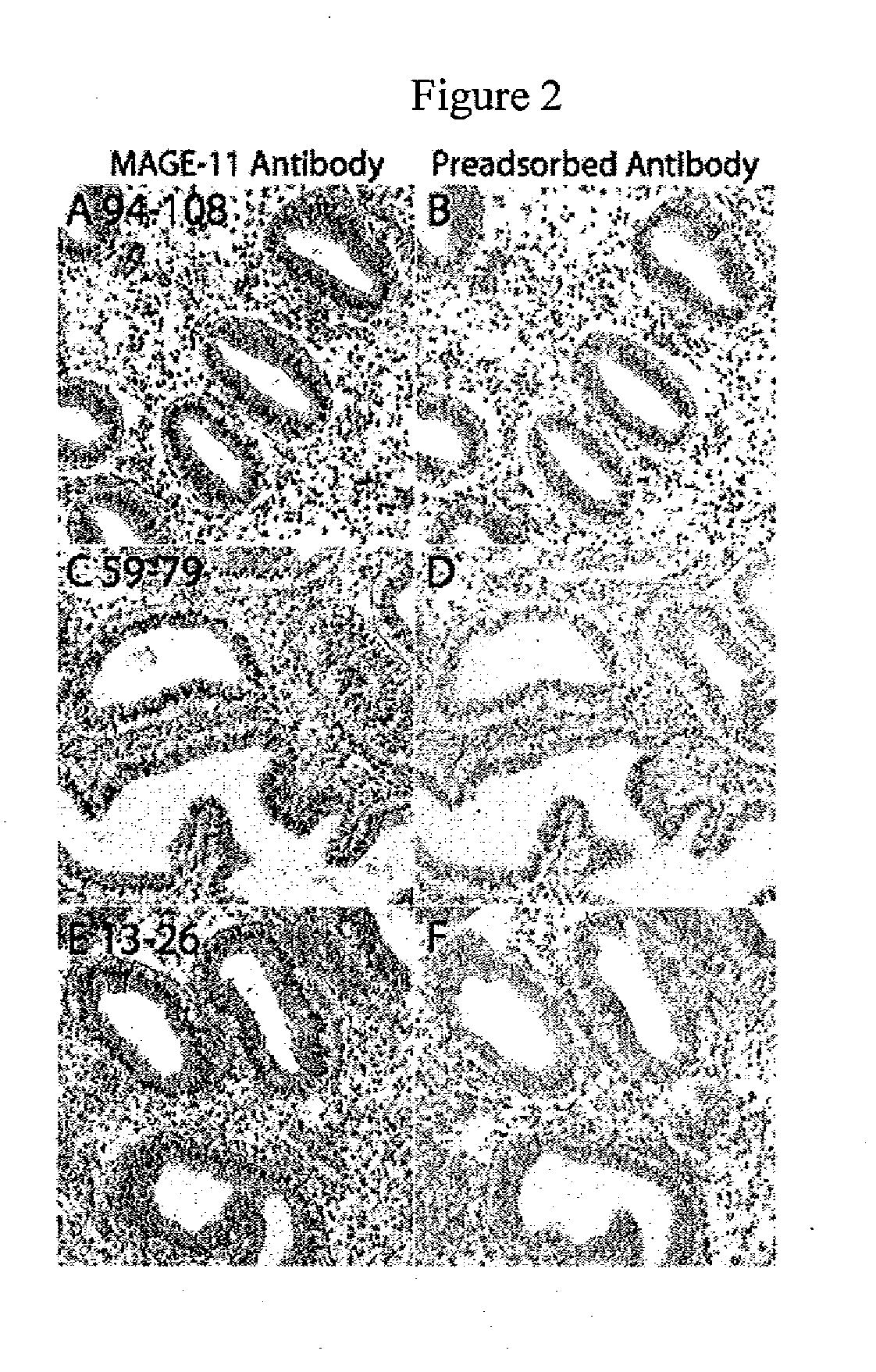
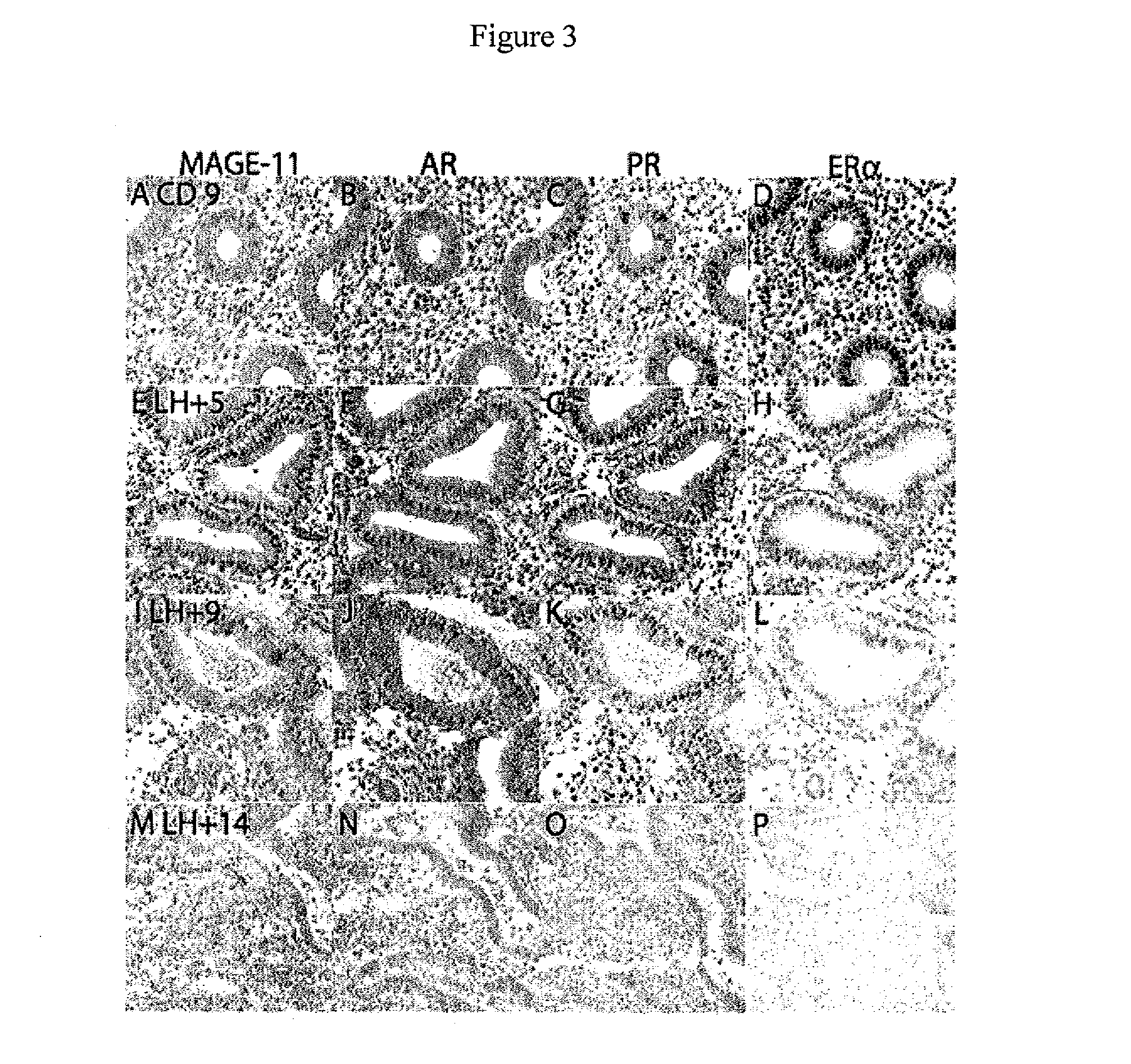
Mage-11 as a marker for endometrial receptivity to embryo transplantation and a marker and therapeutic target in castration-recurrent prostate cancer
Compositions and methods for determining endometrial receptivity to embryo implantation and for detecting and treating castration-recurrent prostate cancer are provided. The methods comprise measuring the level of melanoma antigen gene protein-11 (MAGE-11, also referred to as MAGE-Al 1) in an endometrial or prostate tissue sample. The level of MAGE-11 protein or mRNA can be correlated to endometrial receptivity to embryo implantation in a female human or nonhuman primate, or to the presence of castration-recurrent prostate cancer in a male patient in need thereof. Methods are described whereby MAGE-11 may serve as a target for vaccine development in the treatment of castration-recurrent prostate cancer. Methods for monitoring endometrial maturation, for diagnosing infertility, and for in vitro fertilization in a female human or nonhuman primate are also provided. Compositions of the invention include antibodies that specifically bind MAGE-11 and oligonucleotide primers useful for detecting MAGE-11 mRNA, as well as kits containing such antibodies or primers.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
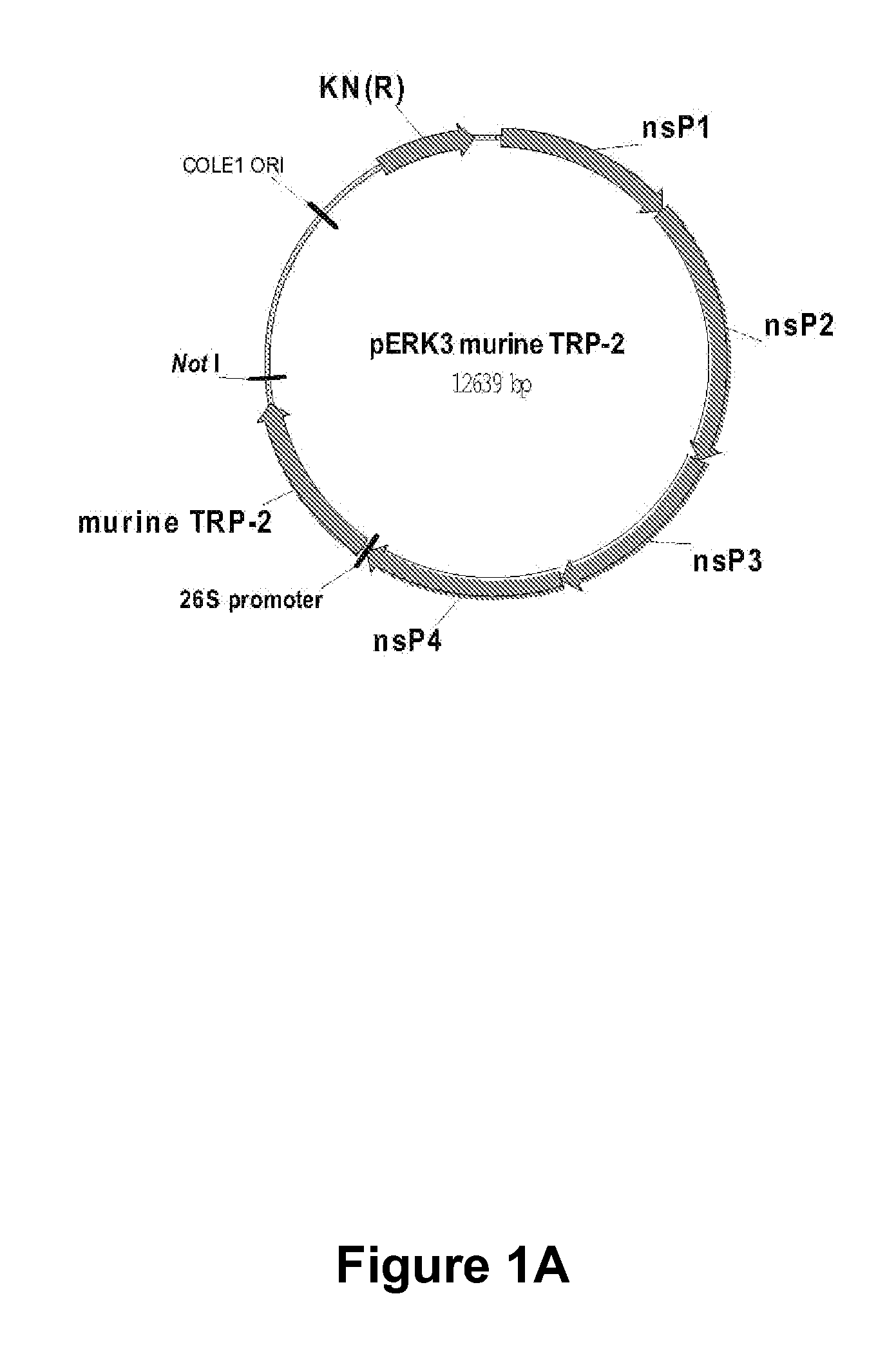
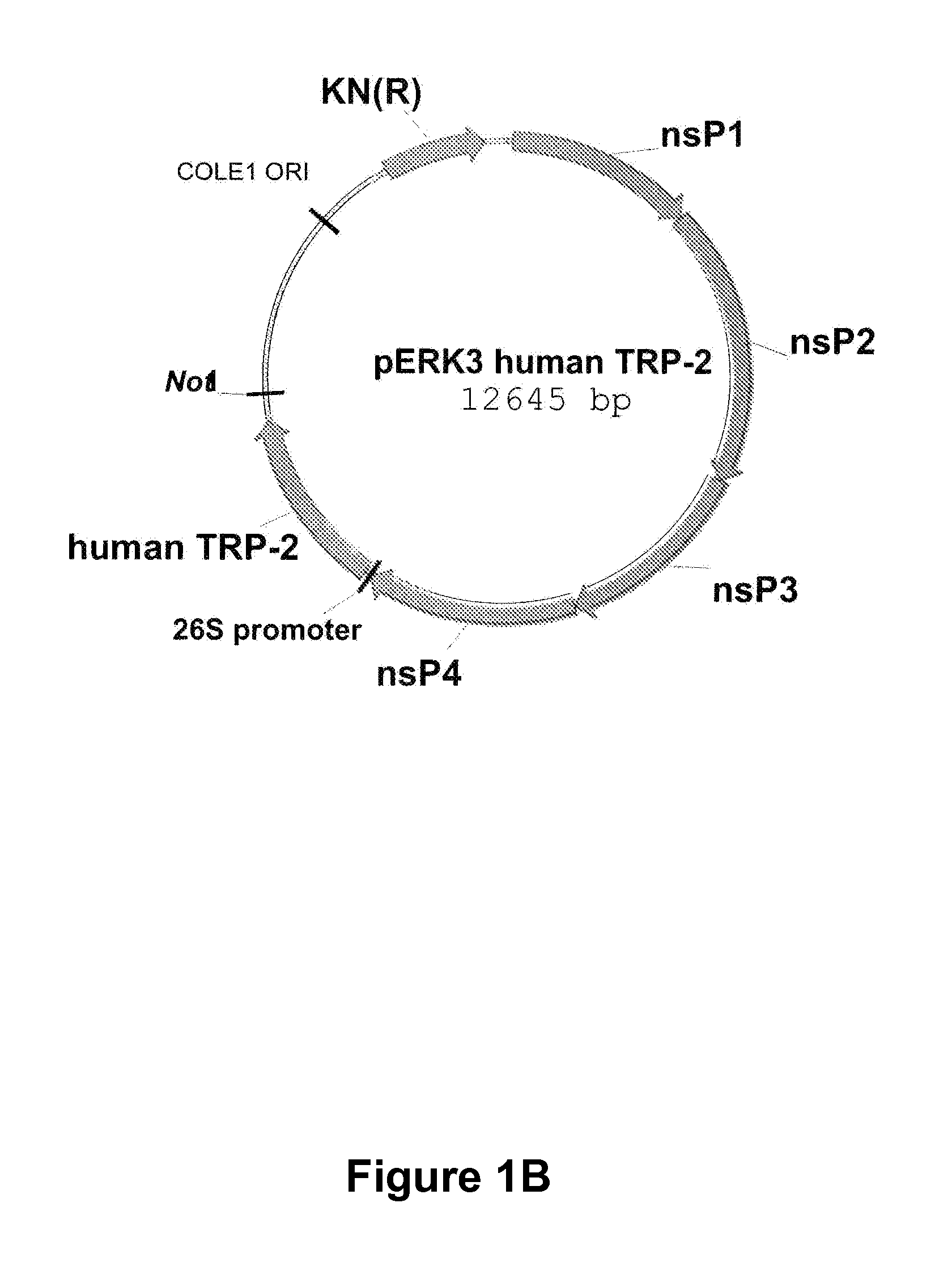
Alphavirus Replicon Particles Expressing TRP2
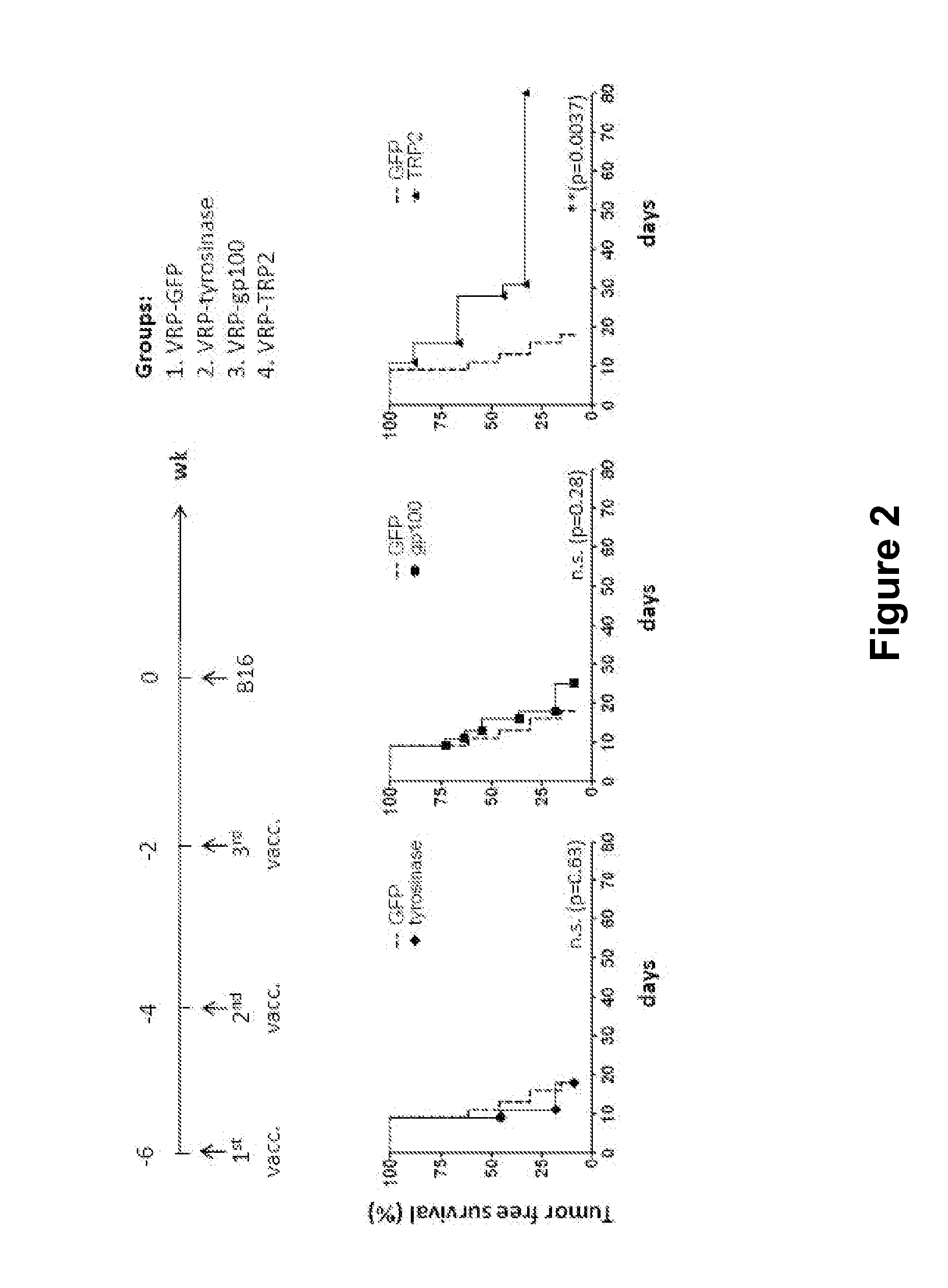
InactiveUS20120128714A1Reduce the possibilityProlong survival timeViral antigen ingredientsCancer antigen ingredientsAdjuvantLymphatic Spread
The immune response to melanoma cells and tumors can be induced or significantly increased by administering to a subject a pharmaceutical composition comprising alphavirus particles, especially Venezuelan equine encephalitis virus replicon particles, which express the melanoma antigen dopachrome tautomerase (DCT, TRP2) in cells of the subject, with the result of tumor regression and / or inhibition of metastasis of a melanoma subject, or a decreased risk of the occurrence or recurrence of melanoma and / or decreased severity of melanoma in a subject not suffering from melanoma at the time of administration. The pharmaceutical composition described herein can be used in conjunction with other therapeutic agents, it can be administered on more than one occasion and it can be combined with administrations of other compositions such as protein or other immunogenic compositions, and / or adjuvants, with beneficial effects to the human or animal subject to which it has been administered.
Owner:ALPHAVAX INC +1
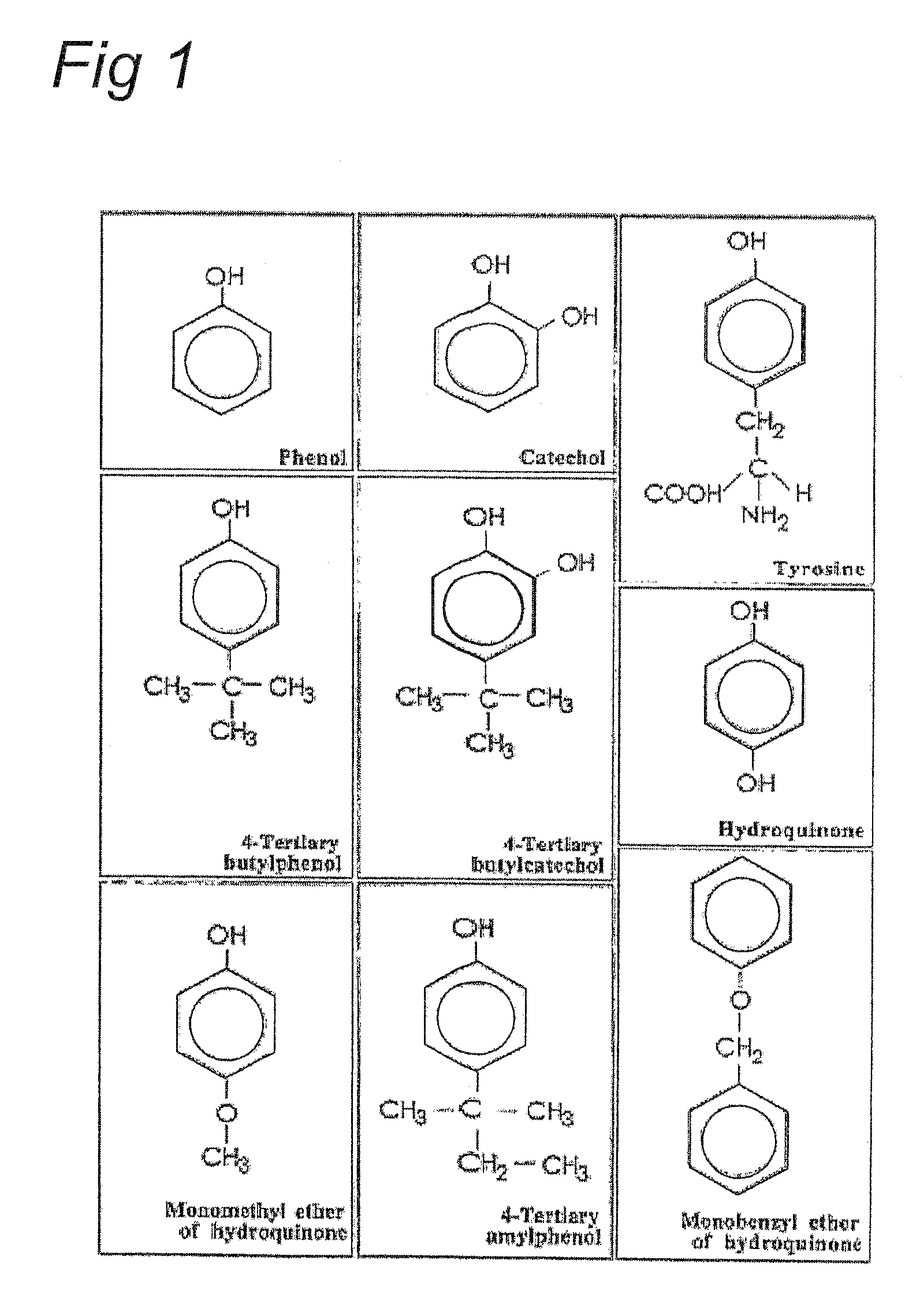
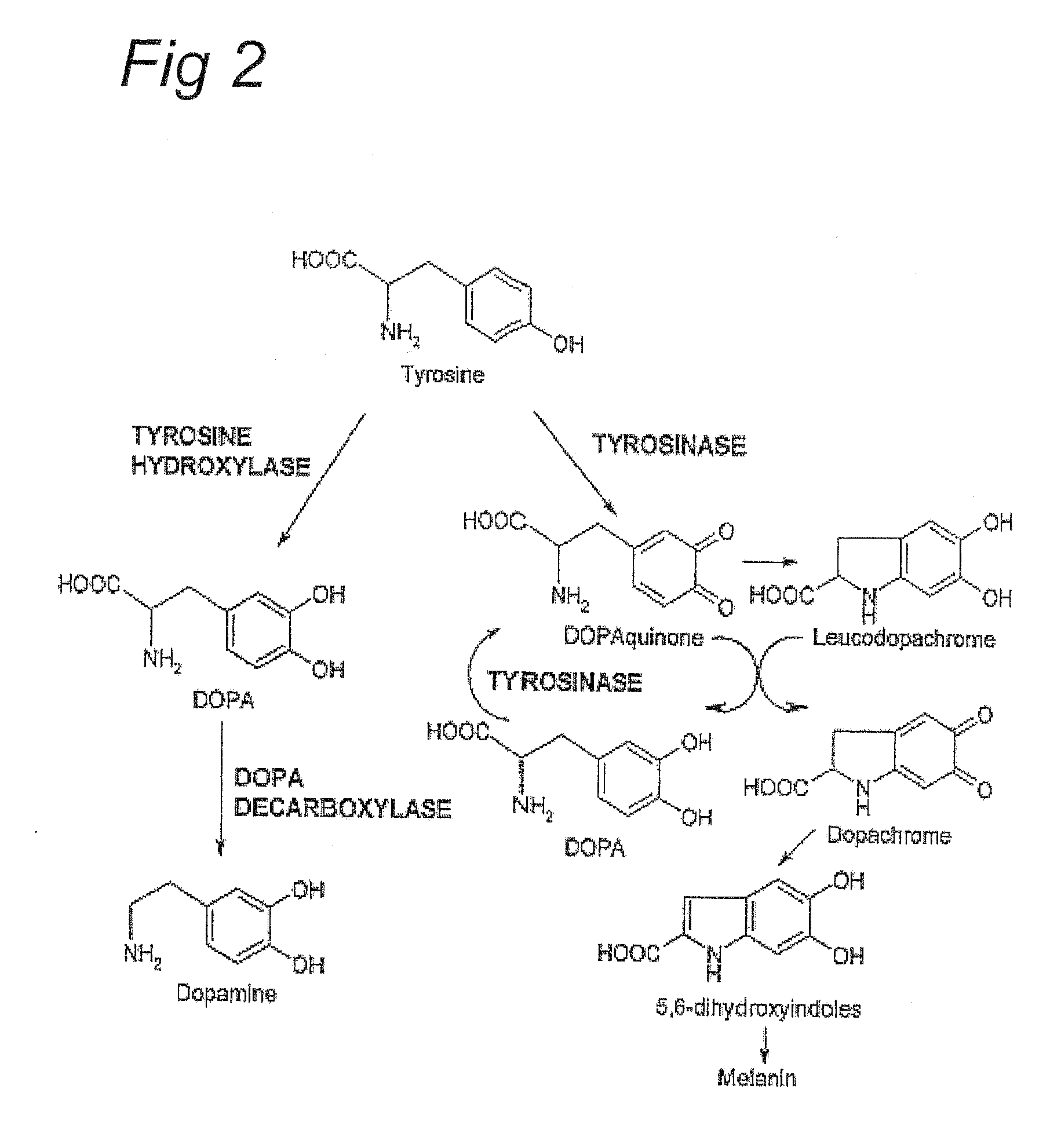
Sensitization of immune system against haptenized melanoma antigens
ActiveUS20120315304A1Reduced activityReduce presencePeptide/protein ingredientsHydroxy compound active ingredientsAntigenProtein oxidation
The metabolization of certain phenols, monophenols or benzenediols into reactive quinone compounds, in particular ortho-quinones and related reactive intermediates, which is brought about by oxidation of monophenols and benzenediols by proteins exhibiting tyrosinase activity, such as human tyrosinase and the related proteins TRP1 and TRP2. The compounds function as haptens that become covalently bound to the tyrosinase enzymes, in particular to histidine moieties, in or near the catalytic site of proteins exhibiting tyrosinase activity, such as tyrosinase, TRP1 and TRP2. An immune response is then to be mounted against these haptenized auto-antigens to treat malignancies.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
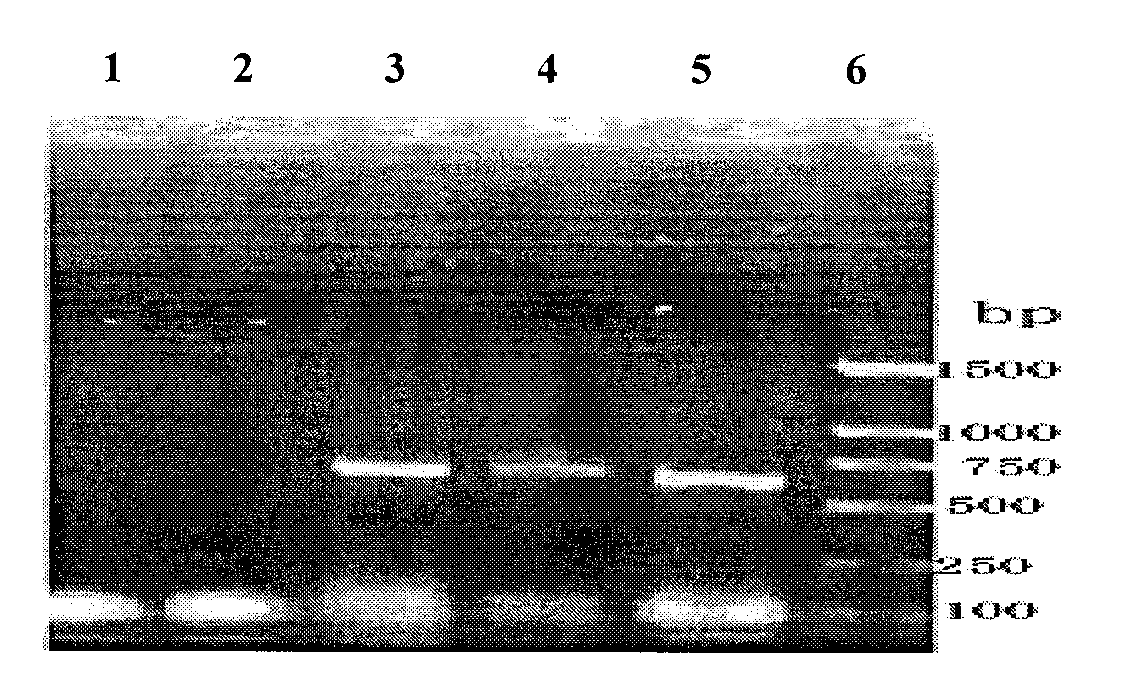
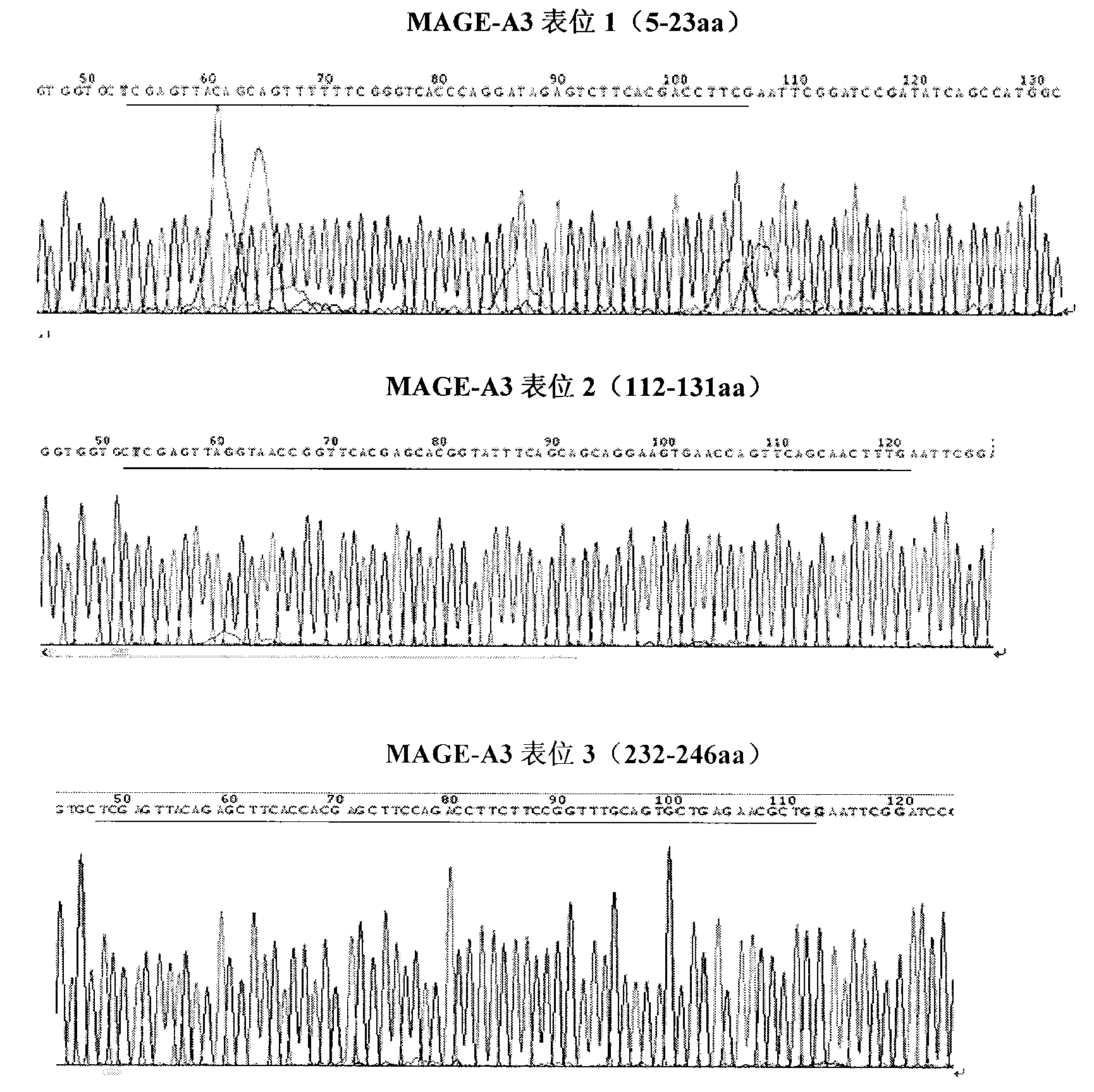
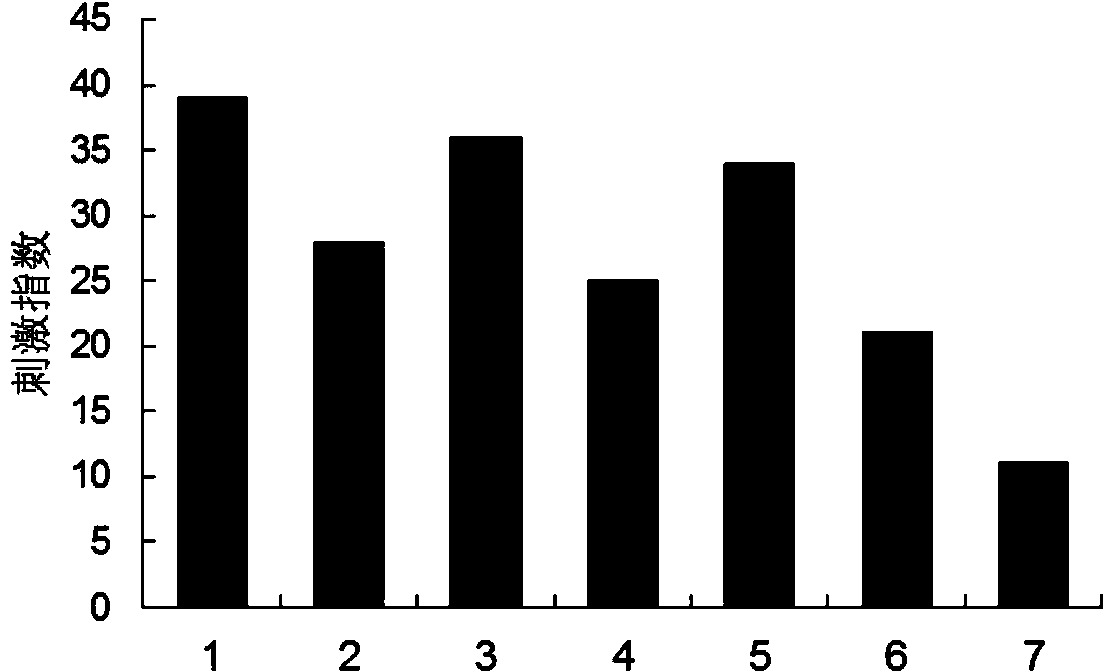
B-cell epitope of human melanoma antigen MAGE-A3 and application thereof
The invention relates to a preparation method and application of a B-cell epitope recombinant protein of human melanoma antigen MAGE-A3 protein. The invention discloses three B-cell epitope-containing polypeptides obtained by screening based on the full-length MAGE-A3 protein. The invention further discloses coded amino acids and nucleotide sequences of B-cell epitope-containing polypeptides. The invention also discloses application of B-cell epitope-containing polypeptides on prevention, treatment and diagnosis of MAGE-A3-positive tumors. The B-cell epitope recombinant protein of MAGE-A3 protein is very strong in immunogenicity and antigenicity, and the application prospect is good.
Owner:WENZHOU MEDICAL UNIV
Methods and compositions for heat shock protein mediated immunotherapy of melanoma
The present invention relates to immunotherapeutic compositions comprising an effective amount of a molecular chaperone such as a heat shock protein, preferably hsp70, non-covalently bound to one or more javelinized melanoma antigens and to methods of using the immunotherapeutic compositions to induce an immune response against melanoma in a subject. The immunotherapeutic composition may contain one or more heat shock proteins, such as one or more of hsp70, hsp90, gp96, BiP, and hsp40, and may contain one or more javelinized melanoma antigens.
Owner:ANTIGENICS +1
Malignant melanoma resisting vaccine composition and application thereof
ActiveCN104353067AEasy to useGood treatment effectBacterial antigen ingredientsAntineoplastic agentsDendritic cellTreatment effect
The invention relates to malignant melanoma resisting vaccine composition which is prepared through mixed culture of an antigen mixture and dendritic cell culture, wherein the antigen mixture comprises malignant melanoma antigens and tuberculosis antigens and / or Auer bodies. The vaccine composition can better stimulate specific T cell proliferation inside the body, has very high specificity killing function on targeted malignant melanoma cells, is convenient to use and good in treatment effect and has good application prospect.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
Methods and materials for detecting colorectal cancer and adenoma
InactiveUS20180187270A1Sensitive highEasy diagnosisMicrobiological testing/measurementDNA methylationMammal
The present invention provides methods and materials related to the detection of colorectal neoplasm-specific markers (e.g., markers associated with colorectal cancer, markers associated with adenoma) in or associated with a subject's stool sample. In particular, the present invention provides methods and materials for identifying mammals (e.g., humans) having a colorectal neoplasm by detecting the presence and level of indicators of colorectal neoplasia such as, for example, long DNA (e.g., quantified by Alu PCR) and the presence and level of tumor-associated gene alterations (e.g., mutations in KRAS, APC, melanoma antigen gene, p53, BRAF, BAT26, PIK3CA) or epigenetic alterations (e.g., DNA methylation) (e.g., CpG methylation) (e.g., CpG methylation in coding or regulatory regions of bmp-3, bmp-4, SFRP2, vimentin, septin9, ALX4, EYA4, TFPI2, NDRG4, FOXE1) in DNA from a stool sample obtained from the mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
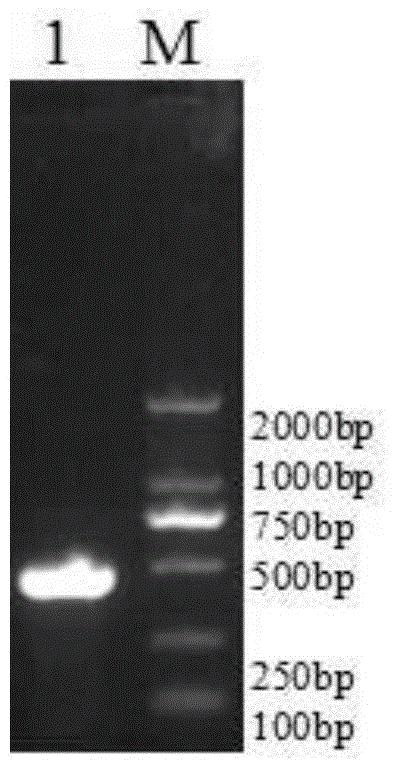
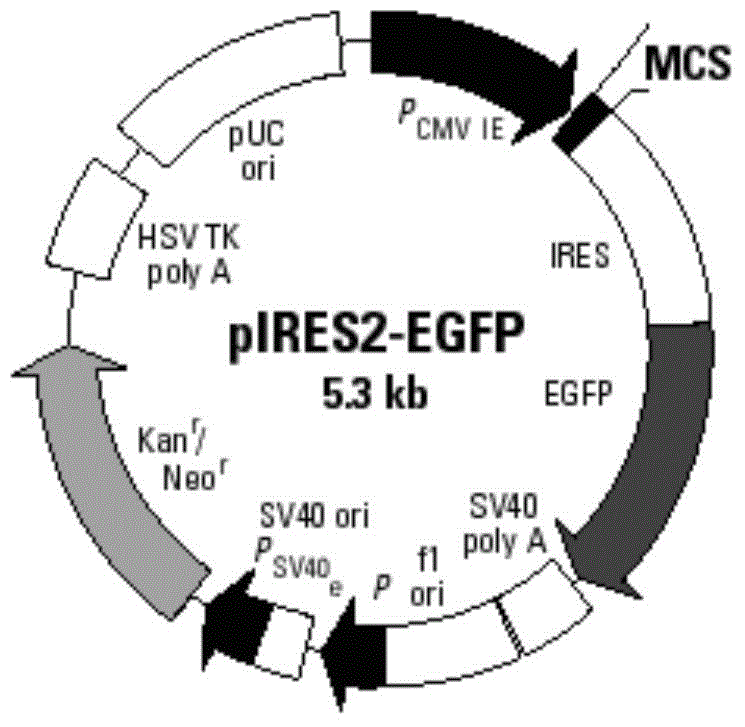
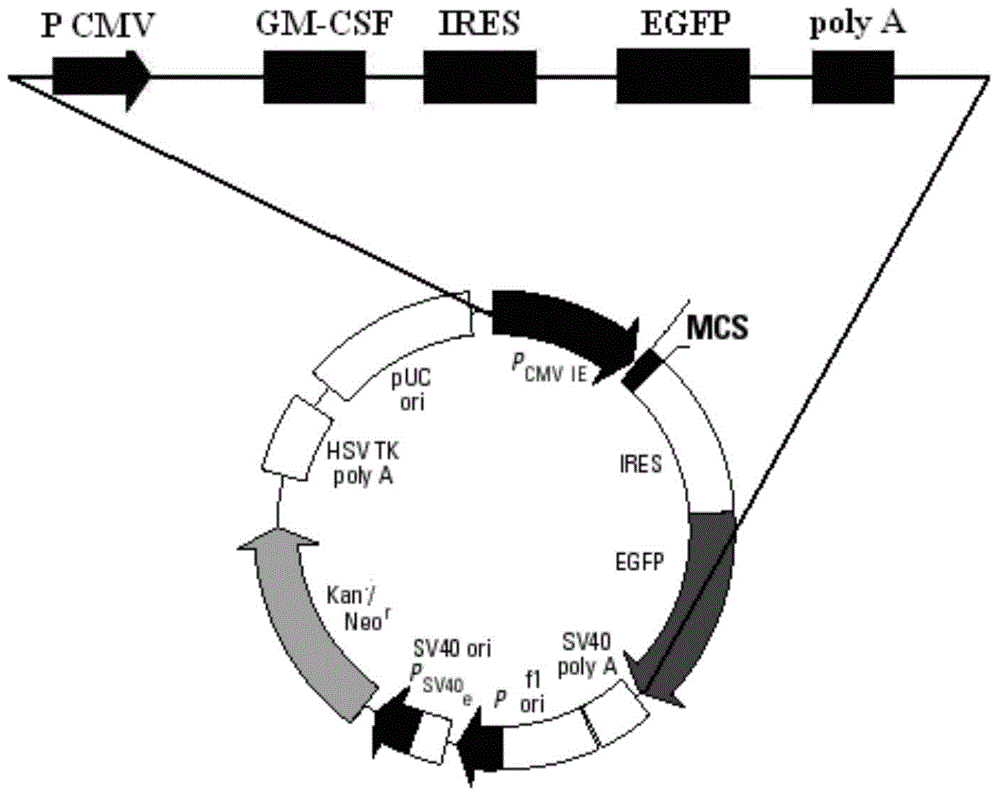
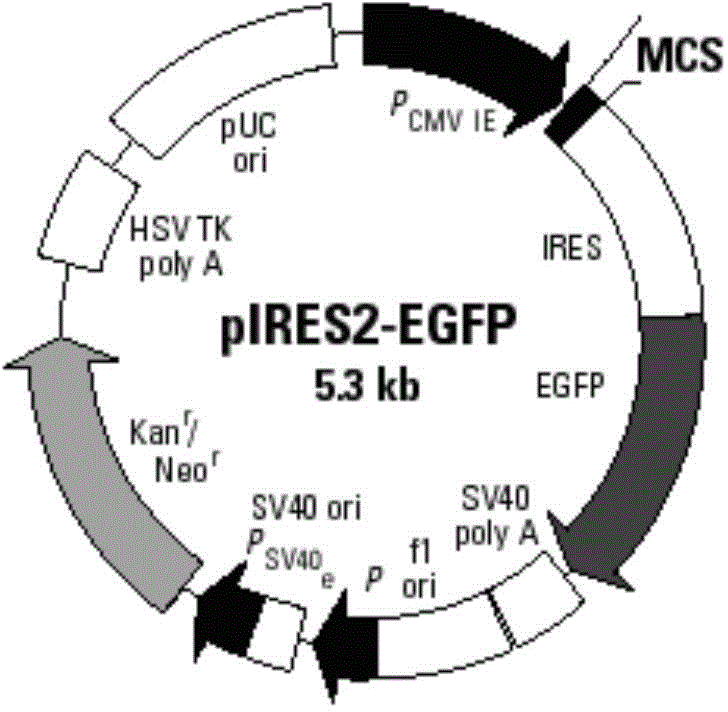
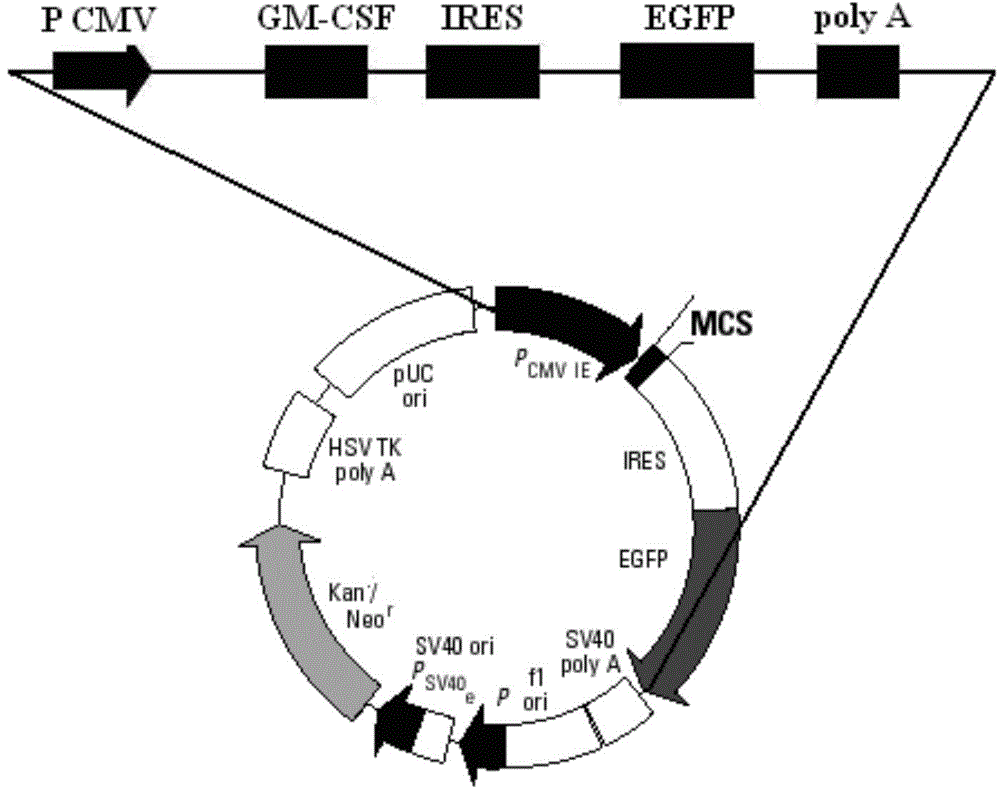
GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) and MART-1 (Melanoma Antigen Recognized By T-Cells 1) dual-gene co-expression recombinant vector and preparation method and application thereof
ActiveCN103602695AGood immunotherapy effectReduce dosageGenetic material ingredientsAntineoplastic agentsAntigenAgricultural science
The invention discloses a GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) and MART-1 (Melanoma Antigen Recognized By T-Cells 1) dual-gene co-expression recombinant vector. The GM-CSF and MART-1 dual-gene co-expression recombinant vector is characterized that a GM-CSF gene, an IRES (Internal Ribosome Entry Sequence) and an MART-1 gene are connected in sequence along the vector transcription direction, or the MART-1 gene, IRES and GM-CSF gene are connected in sequence along the vector transcription direction; a nucleotide sequence of the GM-CSF gene is as shown in SEQ ID NO:1 in a sequence table; the nucleotide sequence of the MART-1 gene is as shown in SEQ ID NO:2 in the sequence table; the nucleotide sequence of the IRES is as shown in SEQ ID NO:3 in the sequence table. According to the dual-gene co-expression recombinant vector, the IRES sequence is utilized for connecting the GM-CSF gene with the MART-1 gene, and thus human melanoma differentiation antigen and the granulocyte-macrophage colony-stimulating factor can be expressed in the same vector at the same time; the recombinant vector can be applied to immunogene therapy of melanoma, enables the immune regulation effect of cytokines to be played, and can also produce specific anti-tumor effect for malignant melanoma in a targeting way.
Owner:HENAN HUALONG BIOLOGICAL TECH
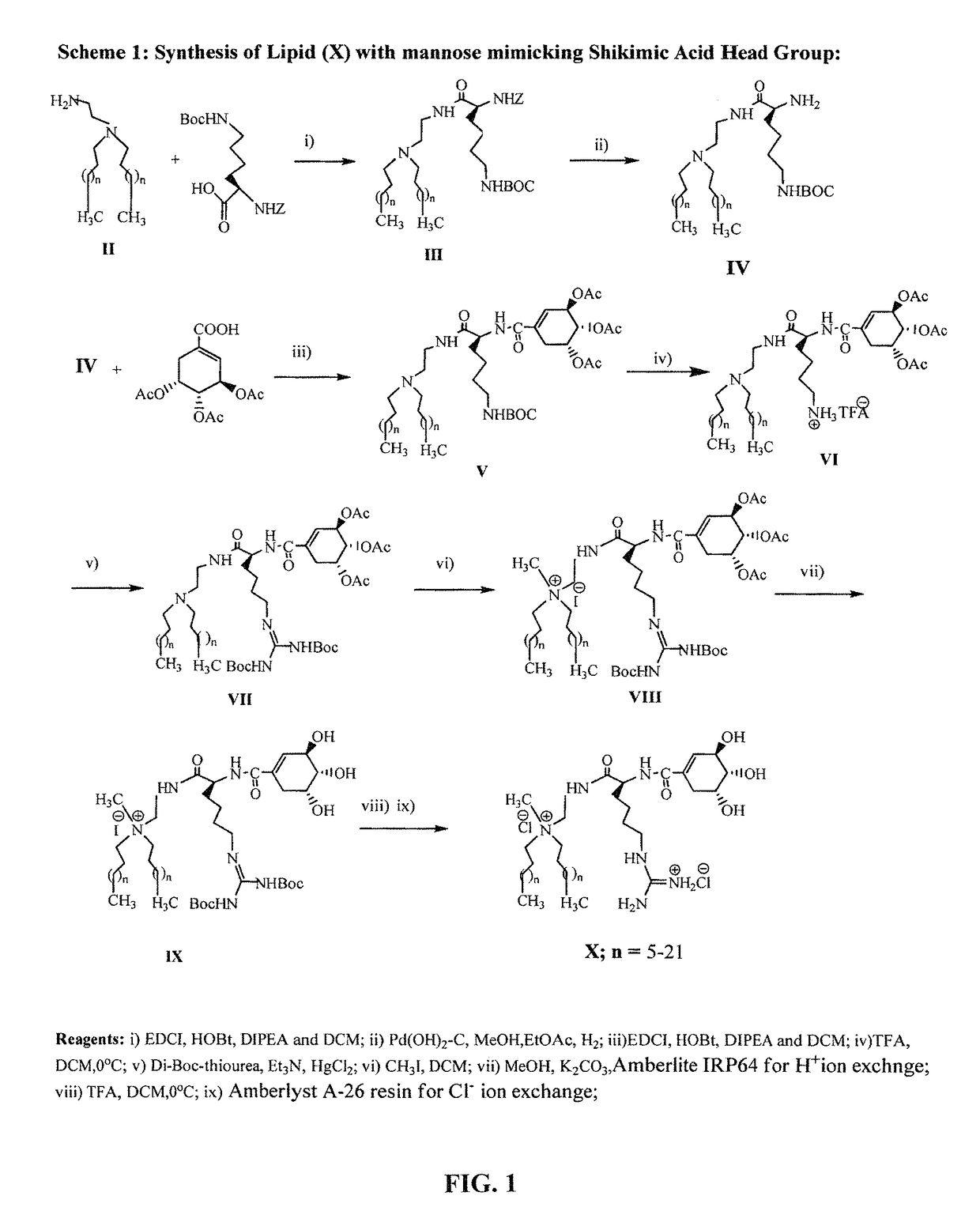
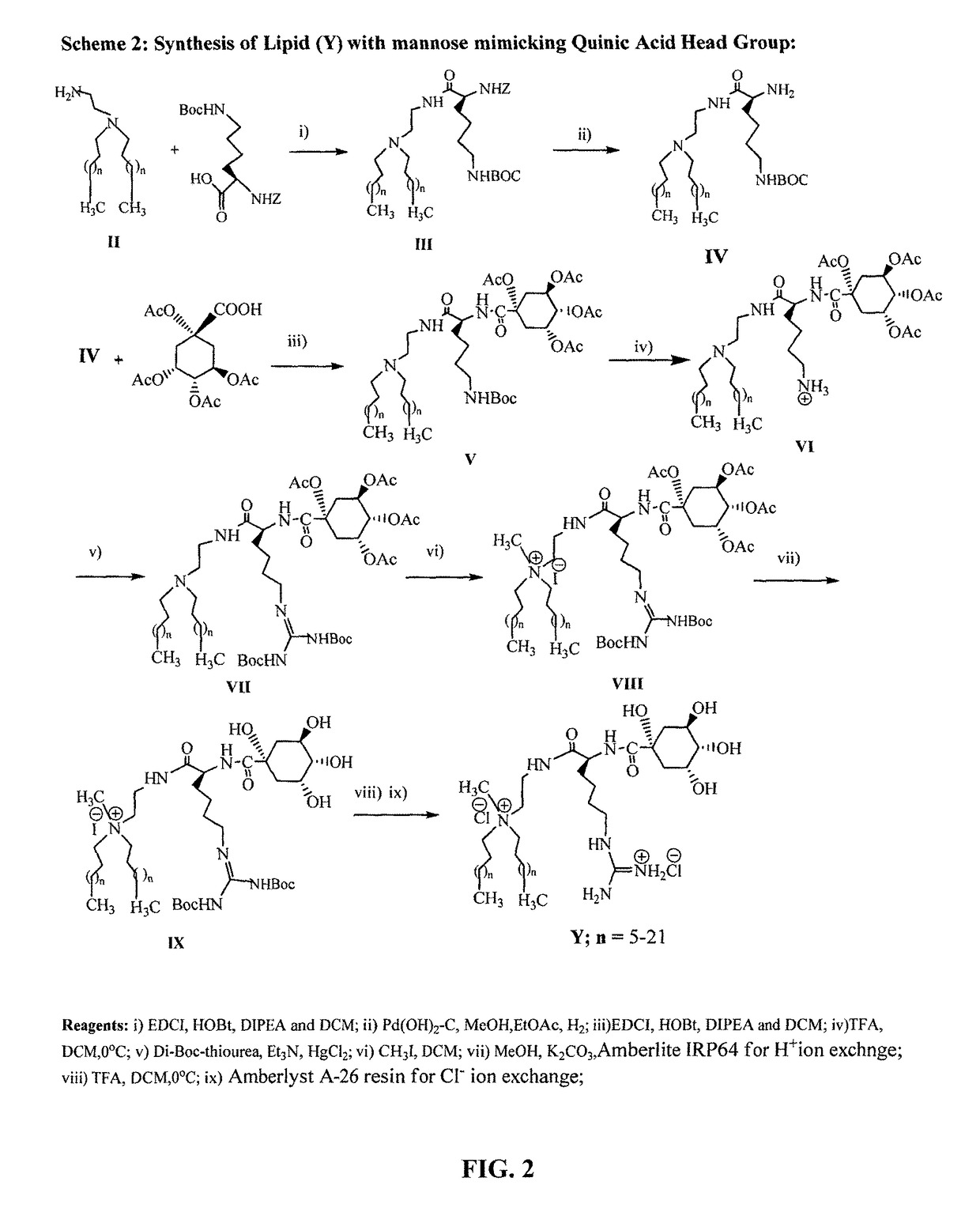
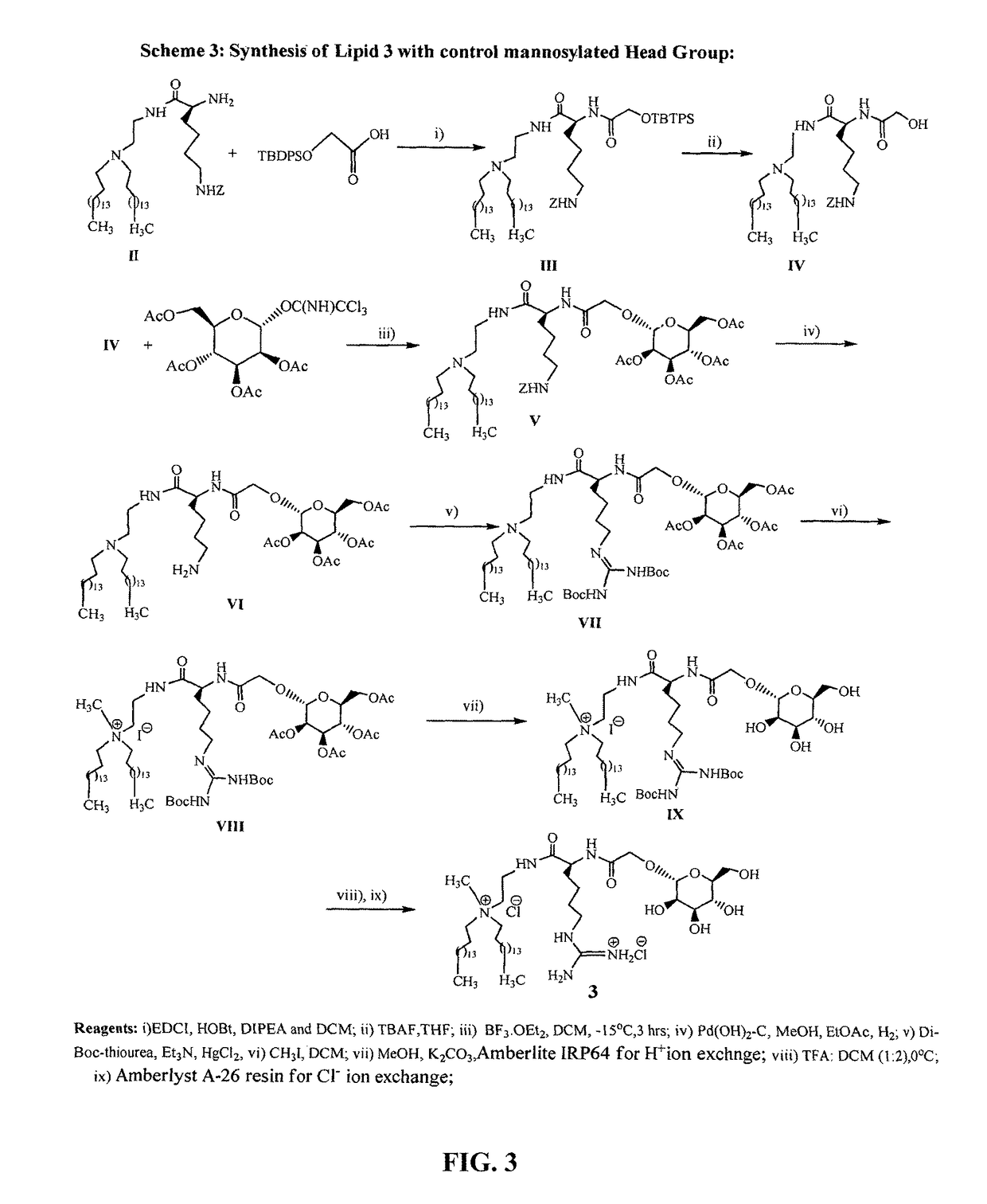
Mannose-receptor selective lysinylated cationic amphiphiles and a process for preparation thereof
ActiveUS9840530B2Efficient deliveryEnhance humoral immune responseEsterified saccharide compoundsOrganic active ingredientsDendritic cellTyrosinase
The present invention relates to the mannose-receptor selective lysinylated cationic amphiphile and a process for preparation thereof. The compounds of the present invention can target DNA vaccines to antigen presenting cells (APCs) such as macrophages and dendritic cells (DCs), via mannose receptors expressed on the cell surface of APCs. The cationic amphiphiles disclosed herein show enhanced cellular and humoral immune response compared to their mannosyl counterparts in genetic immunization in mice. The present invention discloses that immunization with electrostatic complexes (lipoplexes) of DNA vaccines encoding melanoma antigens (gp100 and tyrosinase) and liposome of the presently described novel lysinylated cationic amphiphiles with mannose-mimicking shikimoyl head-groups provides long-lasting (100 days post melanoma tumor challenge) protective immunity in all immunized mice. Cationic amphiphiles with mannose-mimicking shikimoyl head-groups described in the present invention are likely to find future applications in the field of genetic immunization.
Owner:COUNCIL OF SCI & IND RES
Improved targeted t-cell therapy for treatment of multiple myeloma
PendingUS20220062342A1Improve abilitiesLong responseMammal material medical ingredientsCancer antigen ingredientsXBP1Plasma cell
Provided herein are activated adoptive T-cell compositions targeting plasma cell dyscrasias such as multiple myeloma and methods of treating plasma cell dyscrasias such as multiple myeloma using such compositions. The T-cell compositions of the present disclosure are activated against a select group of antigens associated with multiple myeloma (MMAAs) and, in certain embodiments, in combination with more widely expressed tumor associated antigens (TAAs). In particular, the T-cell compositions of the present disclosure are directed to the MMAAs selected from B-cell maturation antigen (BCMA), X box Protein 1 (XBP1), CS1, and Syndecan-1 (CD 138), or a combination thereof. In certain embodiments, the T-cell composition includes T-cells activated to a TAA selected from preferentially expressed antigen of melanoma (PRAME), Survivin, Wilms' Tumor 1 protein (WT1), and melanoma antigen 3 (MAGE-A3), or a combination thereof.
Owner:CHILDRENS NAT MEDICAL CENT
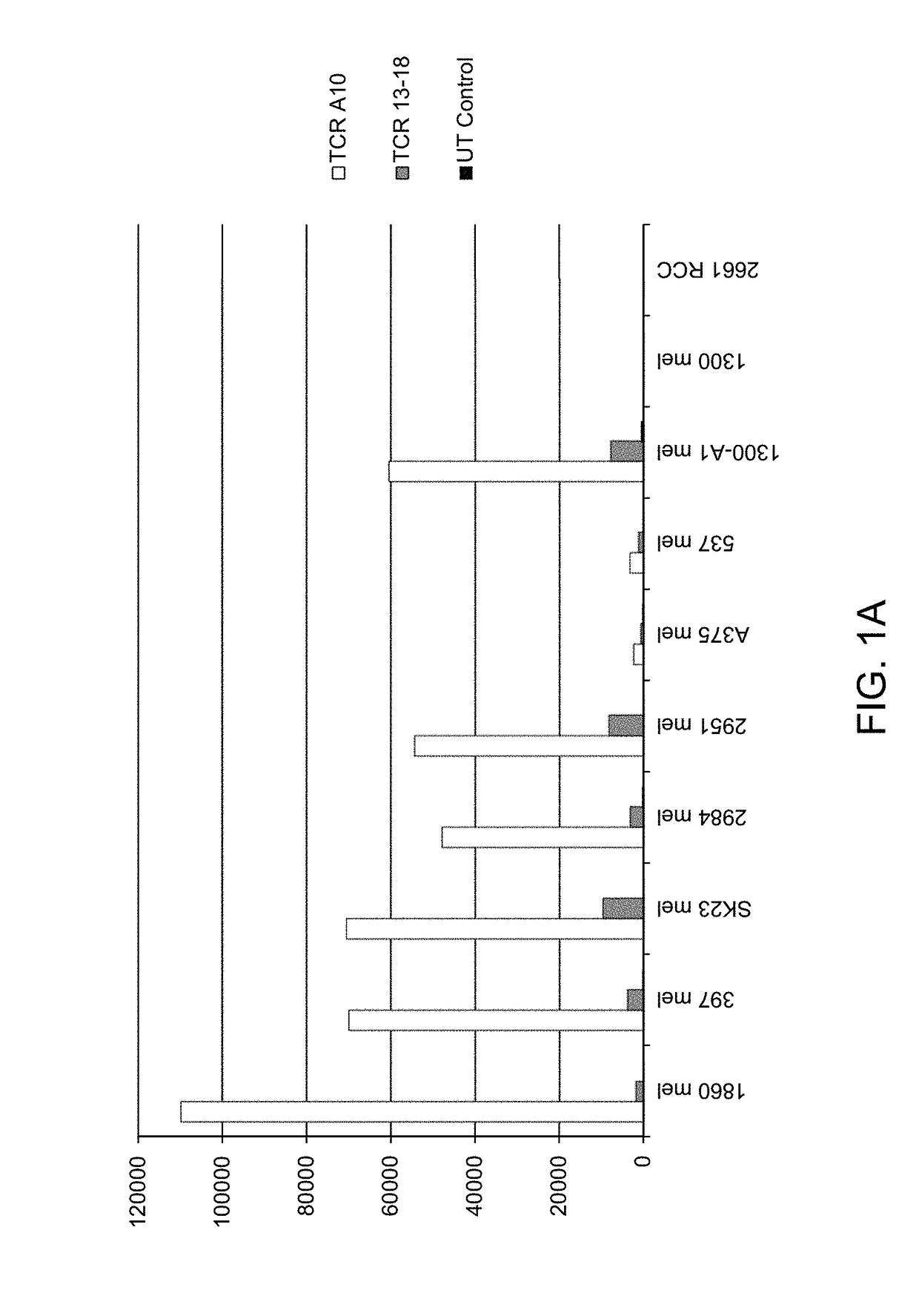
High avidity antigen recognizing constructs
The present invention pertains to novel high avidity antigen recognizing constructs, such as antibodies or T cell receptors, which specifically bind to the melanoma associated antigen (MAGE) A1. The constructs of the invention are particularly useful for the diagnosis, prevention or therapy of tumorous diseases which are characterized by the specific expression of the MAGE-A1 antigen. Furthermore provided are nucleic acids, vectors and host cells—such as CD4 or CD8 positive T cells—which encode, comprise or present the antigen recognizing constructs of the invention. The invention thus provides new means for immune therapy, specifically adoptive T cell therapy, for treating cancer.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
Chimeric antigen receptor with MAGE-A4 specificity and application thereof
The MAGE-A4 or the melanoma associated antigen A4 is a cancer-testicular antigen (CTA) on the X chromosome. The present disclosure provides MAGE-A4 specific chimeric antigen receptors and cells expressing such chimeric antigen receptors. In certain embodiments, engineered cells expressing the chimeric antigen receptors of the present disclosure are capable of inhibiting the growth of MAGE-A4 expressing tumors. The engineered cells of the present disclosure are useful in the treatment of diseases and conditions in which an upregulated or induced MAGE-A4 targeting immune response is desirable and / or therapeutically beneficial. For example, engineered cells expressing the MAGE-A4 specific chimeric antigen receptors of the present disclosure can be used to treat various cancers.
Owner:REGENERON PHARM INC
GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) and MART-1 (Melanoma Antigen Recognized By T-Cells 1) dual-gene co-expression recombinant vector and preparation method and application thereof
ActiveCN103602695BOvercoming reciprocal inhibitionGuarantee structureGenetic material ingredientsAntineoplastic agentsAntigenAgricultural science
The invention discloses a GM-CSF (Granulocyte-Macrophage Colony-Stimulating Factor) and MART-1 (Melanoma Antigen Recognized By T-Cells 1) dual-gene co-expression recombinant vector. The GM-CSF and MART-1 dual-gene co-expression recombinant vector is characterized that a GM-CSF gene, an IRES (Internal Ribosome Entry Sequence) and an MART-1 gene are connected in sequence along the vector transcription direction, or the MART-1 gene, IRES and GM-CSF gene are connected in sequence along the vector transcription direction; a nucleotide sequence of the GM-CSF gene is as shown in SEQ ID NO:1 in a sequence table; the nucleotide sequence of the MART-1 gene is as shown in SEQ ID NO:2 in the sequence table; the nucleotide sequence of the IRES is as shown in SEQ ID NO:3 in the sequence table. According to the dual-gene co-expression recombinant vector, the IRES sequence is utilized for connecting the GM-CSF gene with the MART-1 gene, and thus human melanoma differentiation antigen and the granulocyte-macrophage colony-stimulating factor can be expressed in the same vector at the same time; the recombinant vector can be applied to immunogene therapy of melanoma, enables the immune regulation effect of cytokines to be played, and can also produce specific anti-tumor effect for malignant melanoma in a targeting way.
Owner:HENAN HUALONG BIOLOGICAL TECH
MAGE (Melanoma Antigen Gene)-4 anti-tumor CTL (Cytotoxic T Lymphocyte) epitope peptide and application thereof
The invention relates to an MAGE (Melanoma Antigen Gene)-4 anti-tumor CTL (Cytotoxic T Lymphocyte) epitope peptide. The epitope peptide is a nonapeptide, and the sequence of the nonapeptide is a-b-Leu-Glu-His-Val-Val-Arg-c, wherein a is Lys or Tyr, b is Val or Leu, and c is Val or Leu; the sequence of an epitope peptide P286 is Lys-Val-Leu-Glu-His-Val-Val-Arg-Val; the sequence of an anti-tumor CTL epitope peptide P286-1Y2L is Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Val; and the sequence of an anti-tumor CTL epitope peptide P286-1Y2L9L is Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Leu. The specific CTL induced in the body of a transgenic mouse by the anti-tumor CTL epitope peptide has certain kill rate to tumor cells EC-9706; and the epitope peptide can be used for preparing therapeutic tumor polypeptide vaccines.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
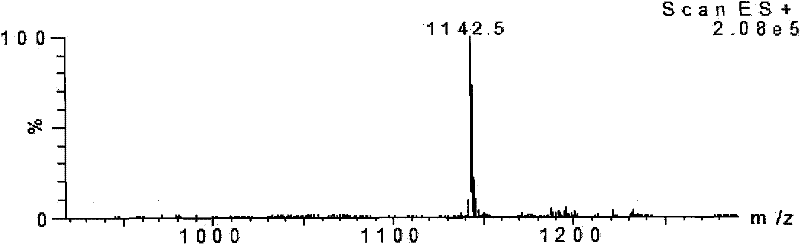
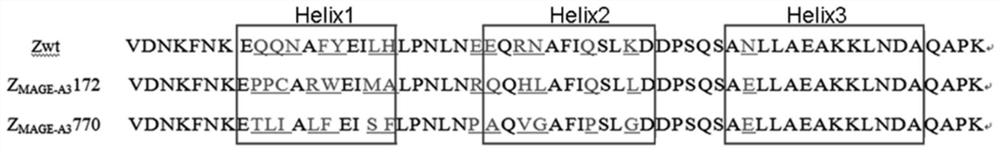
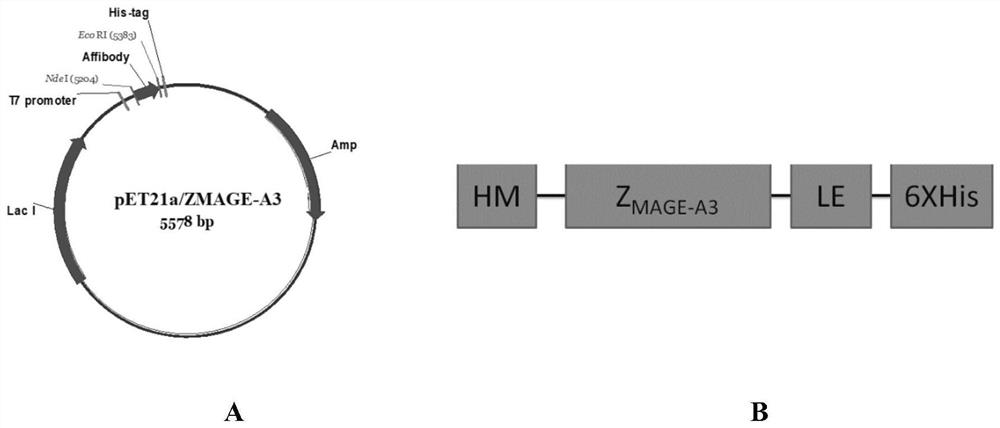
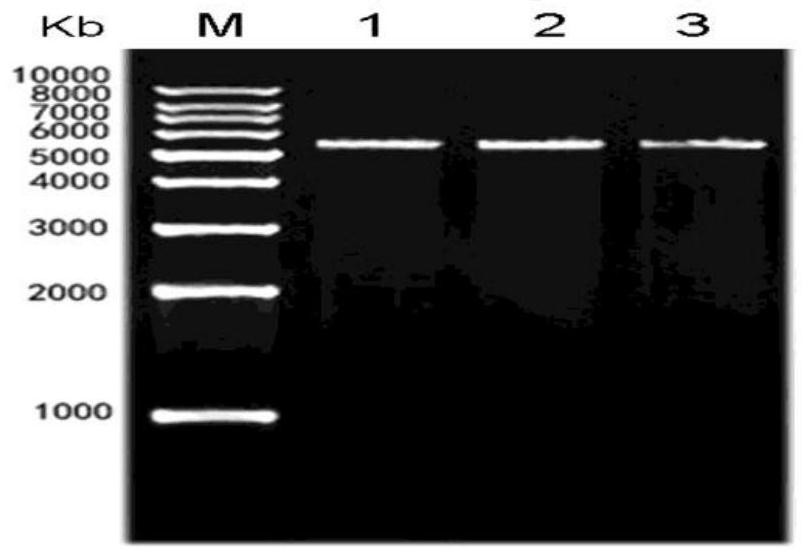
Polypeptide with binding affinity to human melanoma antigen A3 protein and use of polypeptide
The invention relates to a polypeptide with binding affinity to human melanoma antigen MAGE-A3 and use of the polypeptide. The polypeptide with binding affinity to the MAGE-A3 protein is disclosed forthe first time; and the invention also provides application of the polypeptide in diagnosis and detection, and the use of the polypeptide as a targeting carrier in the diagnosis or treatment of drugsor molecular targeting reagents.
Owner:WENZHOU MEDICAL UNIV
In-situ hybridization detection kit of MAGEA3 (Melanoma-Associated Antigen Antiger 3) as well as detection method and application thereof
InactiveCN101988096AHigh sensitivityStrong specificityMicrobiological testing/measurementDiseaseAntigen
The invention relates to an in-situ hybridization detection kit of MAGEA3 (Melanoma-Associated Antigen Antiger 3), comprising a hybridization probe and a marker, wherein the hybridization probe sequence is shown in SEQ ID No.1. The invention also provides an in-situ hybridization detection method of the MAGEA3. In addition, the invention also provides application of the kit to the preparation of medicaments for detecting liver cancer diseases. The kit provided by the invention has the advantages of high sensitivity and strong specificity; and the detection method of the invention is convenient and simple for operation and can be widely used and popularized in municipal or higher-grade hospitals.
Owner:NATUREGEN BIOTECH SHANGHAI
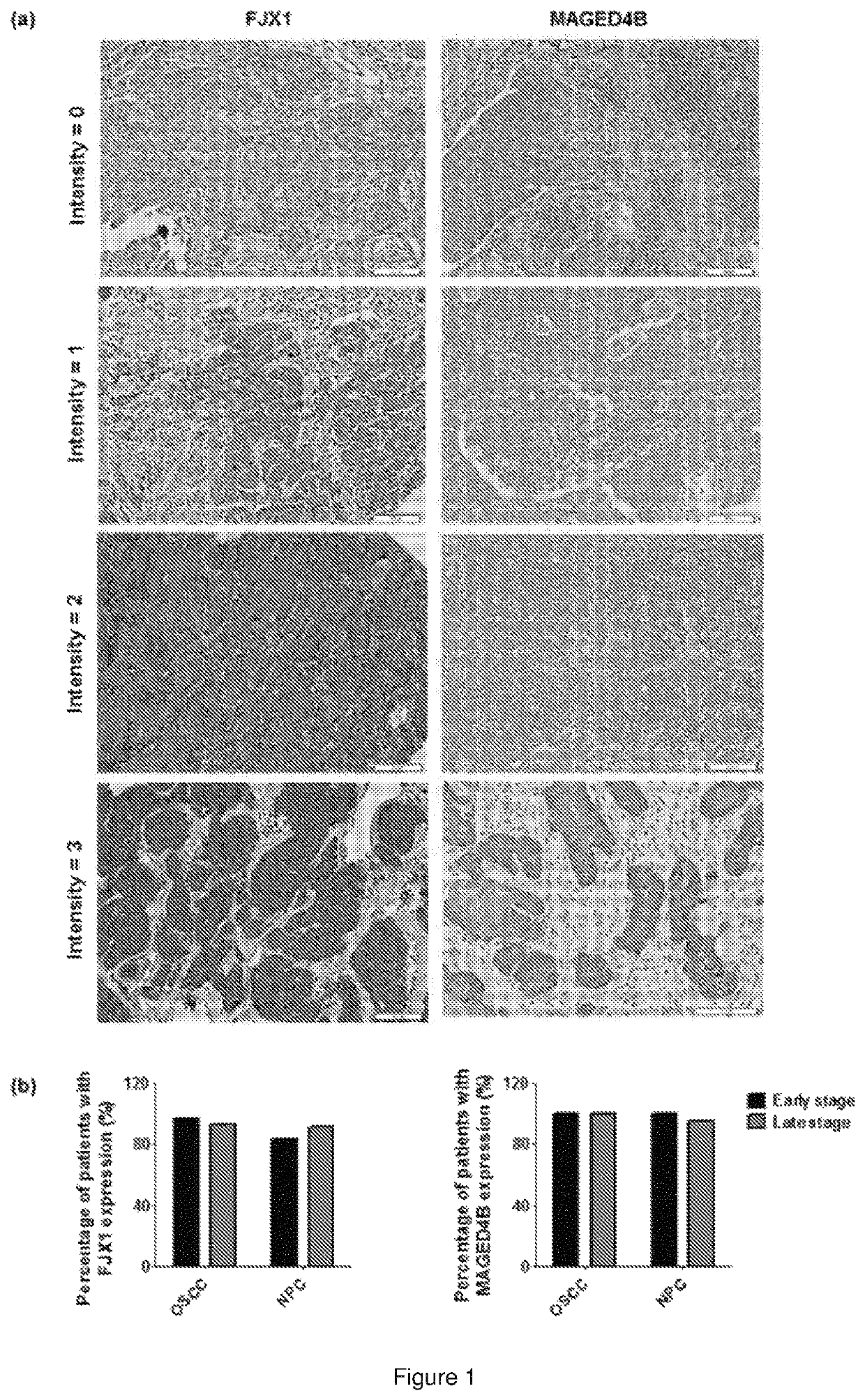
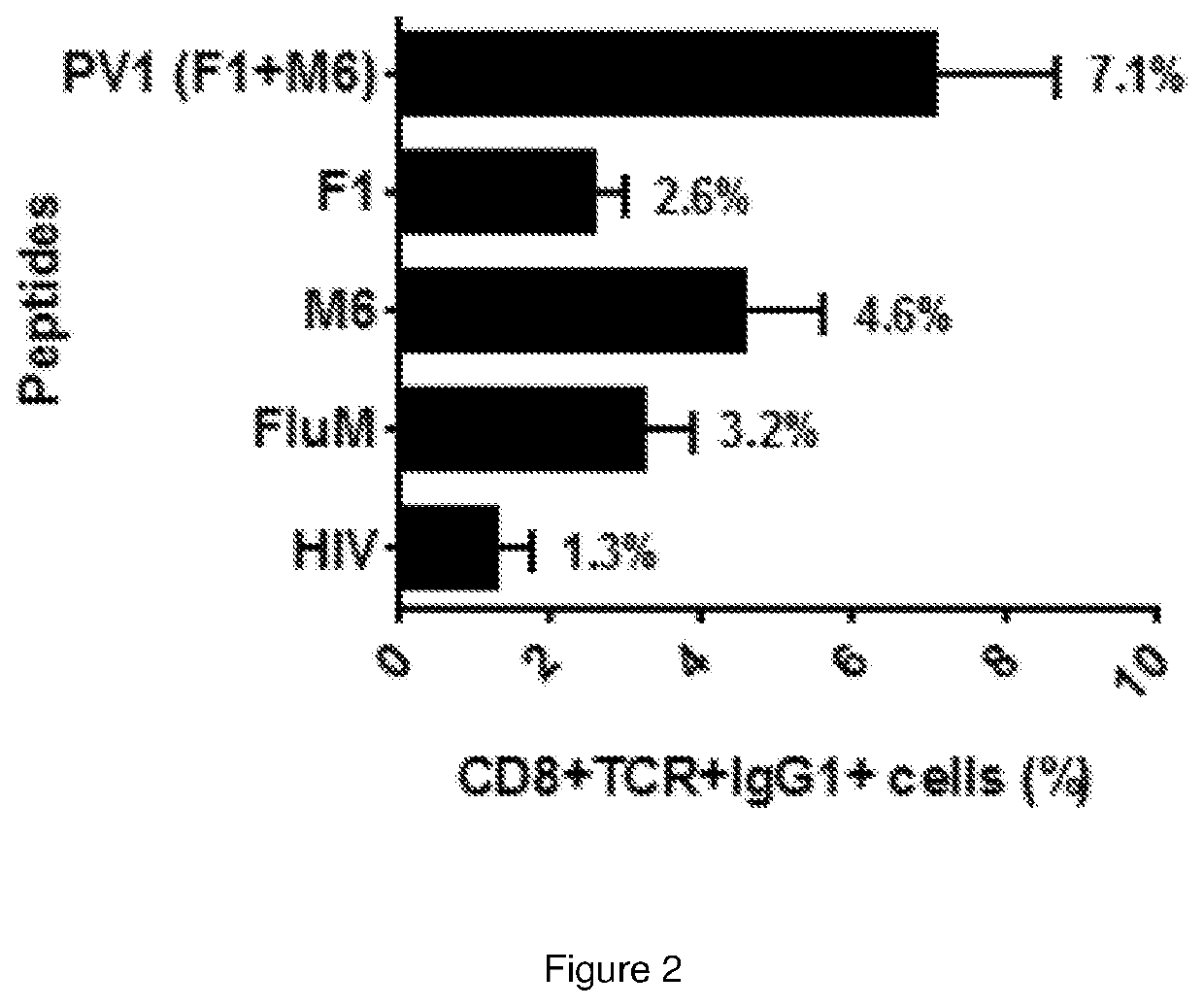
Immunogenic peptide composition
ActiveUS20200138929A1Promote growthAntibody ingredientsCancer antigen ingredientsMajor histocompatibilityMolecular binding
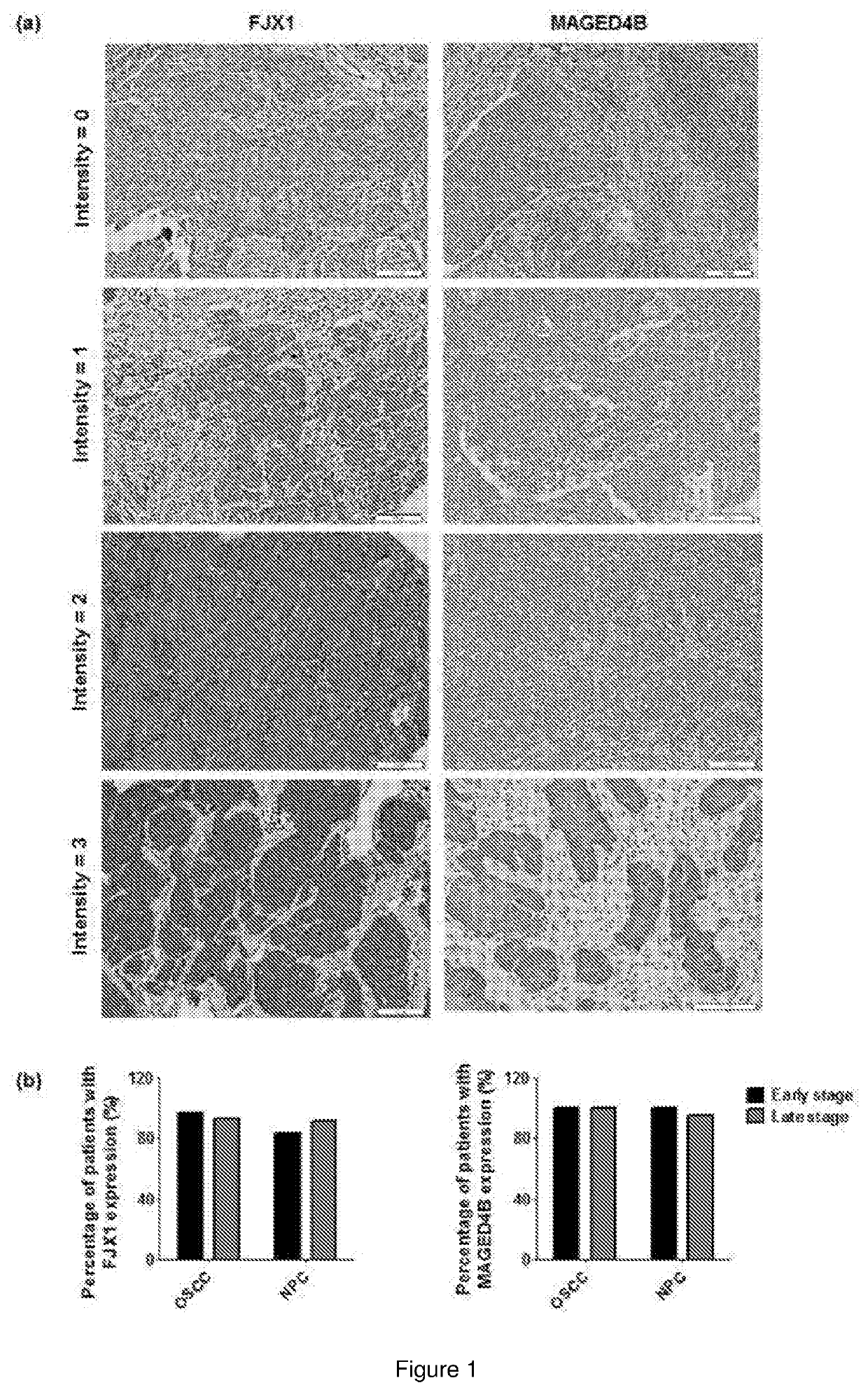
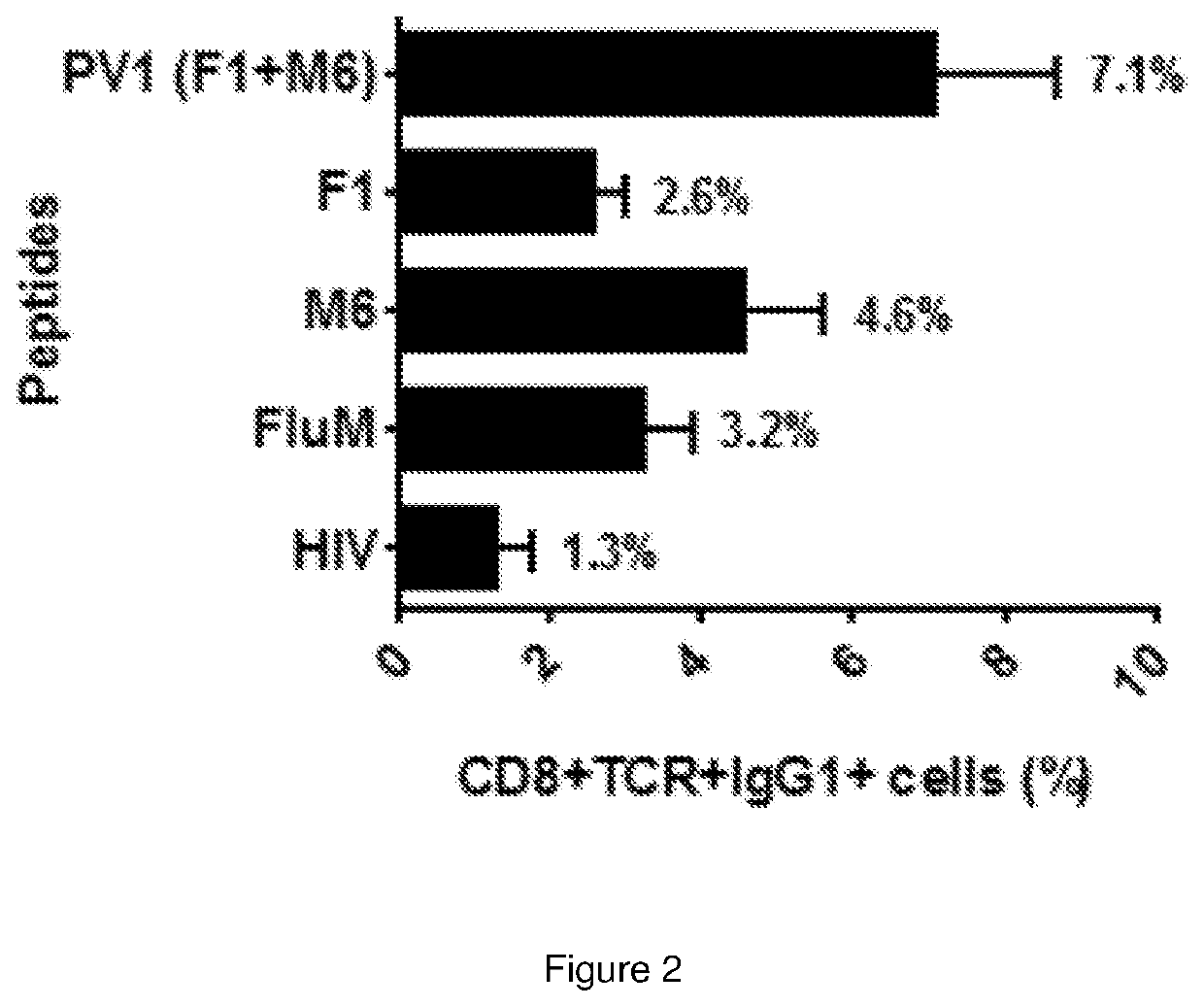
The present invention relates to a peptide composition capable of binding with major histocompatibility complex class I molecules to induce an anti-cancer immune response in a subject. Particularly, the peptide composition comprises at least a Four-jointed Box 1 peptide and a Melanoma Antigen family D4b peptide. The present invention further relates to the use of a peptide composition and a peptide vaccine for inducing the anti-cancer immune response in the subject.
Owner:CANCER RES MALAYSIA
A kind of anti-malignant melanoma vaccine composition and its application
ActiveCN104353067BEasy to useGood treatment effectBacterial antigen ingredientsAntineoplastic agentsMelanoma VaccineDendritic cell
The invention relates to an anti-malignant melanoma vaccine composition, which is prepared by mixed culture of antigen mixture and dendritic cell culture, wherein the antigen mixture includes malignant melanoma antigen, tuberculosis antigen and / or rod-shaped body. The vaccine composition can better stimulate the proliferation of specific T cells in the body, and has a very strong specific killing function on target malignant melanoma cells. The vaccine composition is easy to use, has a good therapeutic effect, and has a good application prospect.
Owner:FIRST HOSPITAL AFFILIATED TO GENERAL HOSPITAL OF PLA
T-cell receptors that recognize short peptides of the mage-a1 antigen
ActiveCN106749620BGood killing effectImmunoglobulin superfamilyPeptide/protein ingredientsAntigenOligopeptide
The invention provides a TCR (T Cell Receptor) which is capable of specifically binding oligopeptides KVLEYVIKV derived from an MAGE-A1 (Melanoma antigen A1). A compound can be formed by the oligopeptides KVLEYVIKV derived from the MAGE-A1 and an HLA (Human Leukocyte Antigen) A0201, and the oligopeptides KVLEYVIKV and the HLA A0201 can be transferred to cell surfaces together. The invention also provides a nucleic acid molecule for coding the TCR and a carrier comprising the nucleic acid molecule. In addition, the invention also provides a cell for transducing the TCR.
Owner:XLIFESC LTD
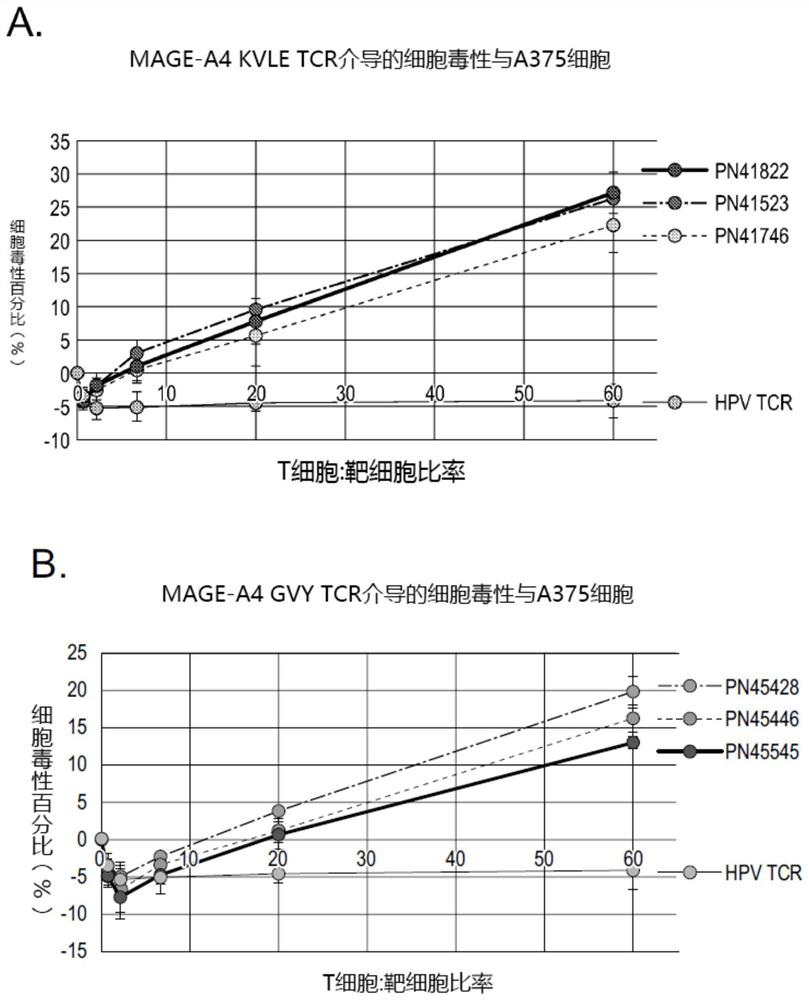
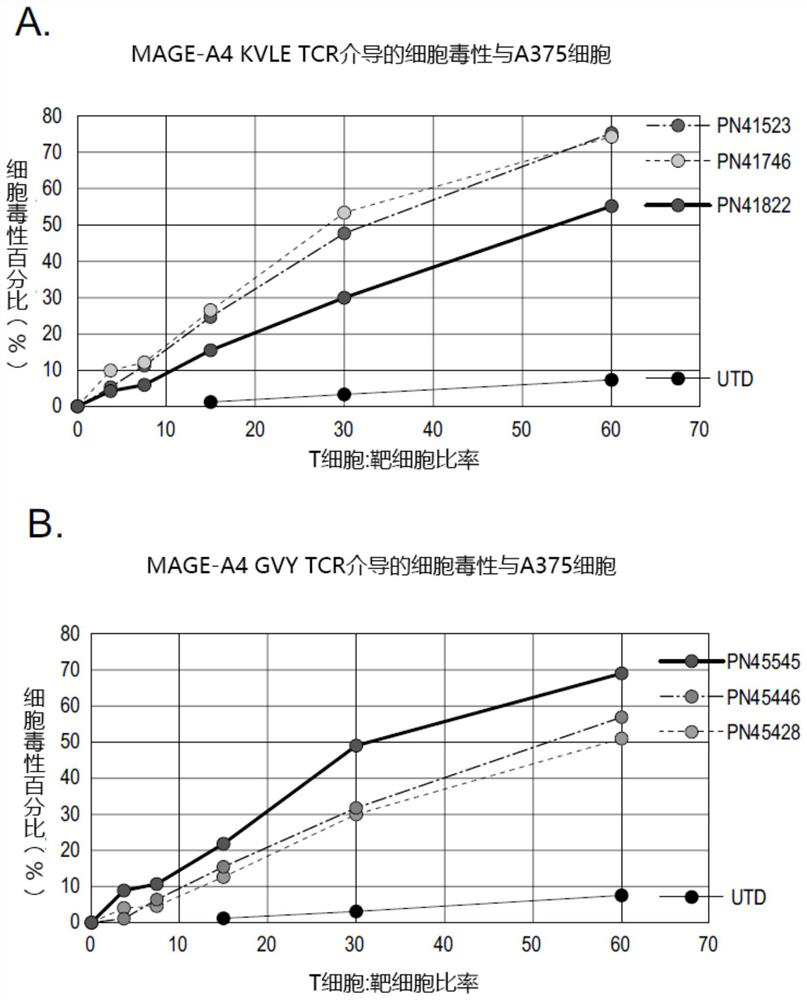
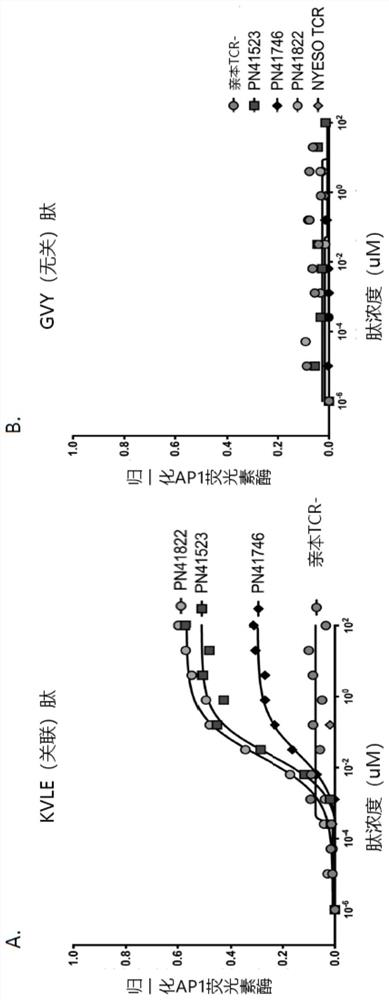
MAGE-A4 T cell receptors and methods of use thereof
PendingCN114585646AImmunoglobulin superfamilyTumor rejection antigen precursorsPeptide antigenOncology
The present invention provides isolated T cell receptors (TCRs) that specifically bind to the HLA presenting cancer-testicular antigen melanoma associated antigen A4 (MAGE-A4) peptide, and therapeutic and diagnostic methods of using these isolated TCRs. The present invention provides a T cell receptor (TCR) produced for an anti-MAGE-A4 peptide antigen in the case of MHC (HLA-A2). In reporter gene detection, the determined unique TCR sequence can be specifically combined with the small peptide MAGE-A4 in the HLA molecular groove, and T cells are activated.
Owner:REGENERON PHARM INC
T cell receptors recognizing hla-a1- or hla-cw7-restricted mage
ActiveUS20190016777A1Immunoglobulin superfamilyPeptide/protein ingredientsAntigen bindingNucleic acid
The invention provides an isolated or purified T cell receptor (TCR) having antigenic specificity for a) melanoma antigen family A (MAGE A)-3 in the context of HLA-A1 or b) MAGE-A12 in the context of HLA-Cw7. The invention further provides related polypeptides and proteins, as well as related nucleic acids, recombinant expression vectors, host cells, and populations of cells. Further provided by the invention are antibodies, or an antigen binding portion thereof, and pharmaceutical compositions relating to the TCRs of the invention. Methods of detecting the presence of cancer in a host and methods of treating or preventing cancer in a host are further provided by the invention.
Owner:UNITED STATES OF AMERICA
Immunogenic peptide composition
ActiveUS11260118B2Promote growthAntibody ingredientsCancer antigen ingredientsMajor histocompatibilityMolecular binding
The present invention relates to a peptide composition capable of binding with major histocompatibility complex class I molecules to induce an anti-cancer immune response in a subject. Particularly, the peptide composition comprises at least a Four-jointed Box 1 peptide and a Melanoma Antigen family D4b peptide. The present invention further relates to the use of a peptide composition and a peptide vaccine for inducing the anti-cancer immune response in the subject.
Owner:CANCER RES MALAYSIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com