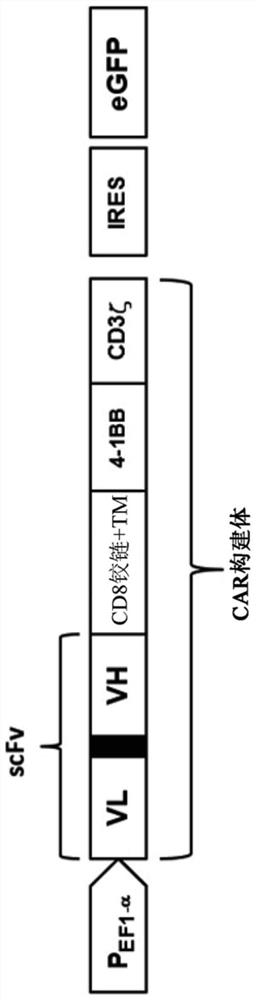
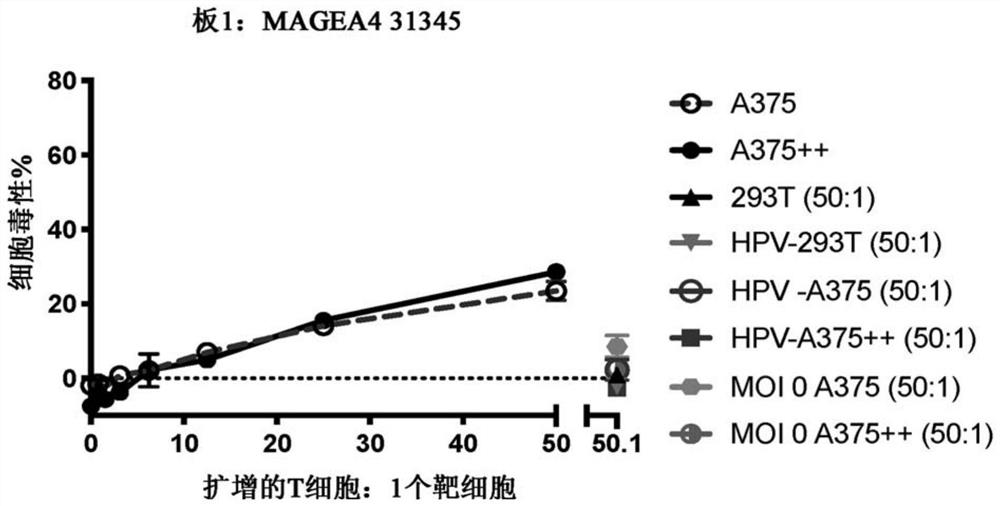
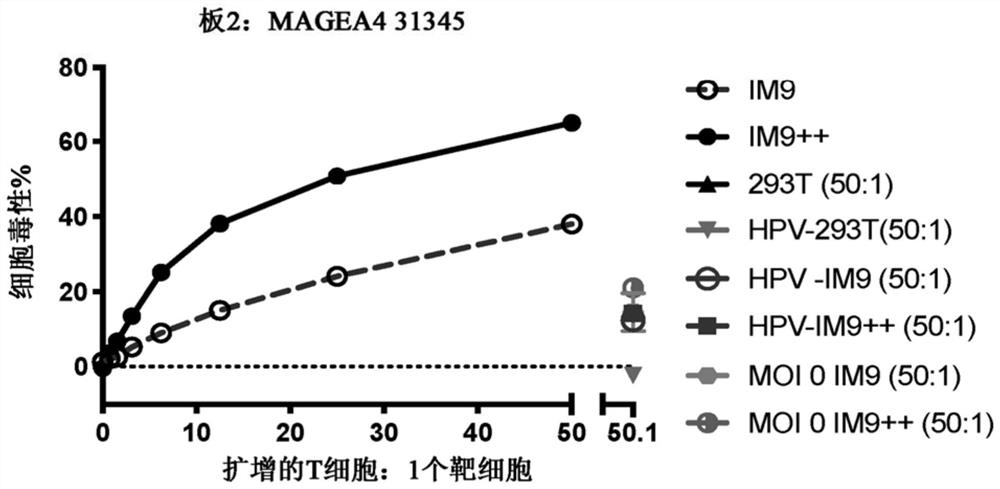
Chimeric antigen receptor with MAGE-A4 specificity and application thereof
A MAGE-A4, chimeric antigen receptor technology, applied in medical preparations containing active ingredients, antibody medical ingredients, DNA/RNA fragments, etc., can solve problems such as unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0144] Preparation of antigen-binding domains
[0145] The antigen binding domains of chimeric antigen receptors of the present disclosure are specific for a particular antigen (eg, MAGE-A4) and can be prepared by any antibody production technique known in the art. In certain embodiments, one or more of the individual components (eg, heavy and light chains) of the corresponding antibodies of the disclosure are derived from chimeric, humanized, or fully human antibodies. Methods for making such antibodies are well known in the art. For example, VELOCIMMUNE can be used TM Techniques One or more of the heavy and / or light chains are produced. Use VELOCIMMUNE TM technology (or any other technique for antibody production by others), high affinity chimeric antibodies are initially isolated to a specific antigen (eg, MAGE-A4) with human variable regions and mouse constant regions. Antibodies are characterized and selected for desired properties including affinity, selectivity, epi...
example
[0202] The following examples are presented to provide those of ordinary skill in the art with a complete disclosure and description of how to make and use the methods and compositions of the disclosure, and are not intended to limit the scope of what the inventors regard as their invention. Efforts have been made to ensure accuracy with respect to numbers used (eg, amounts, temperature, etc.), but some experimental errors and deviations should be accounted for. Unless indicated otherwise, parts are parts by weight, molecular weight is average molecular weight, temperature is in degrees Celsius, and pressure is at or near atmospheric.
example 1
[0203] Example 1: Generation of anti-MAGE-A4 antibodies
[0204] Genetically modified mice (e.g., including human immunoglobulins encoding Anti-MAGE-A4 antibodies were obtained by engineered mice with DNA of variable region of protein heavy chain and κ light chain.
[0205] After immunization, splenocytes were harvested from each mouse and either (1) fused with mouse myeloma cells to maintain their viability and form hybridomas and screened for MAGE-A4 specificity or (2) using human MAGE-A4 fragments B cells are sorted as a sorting reagent that binds and identifies reactive antibodies (antigen positive B cells) (as described in US Patent Publication No. 2007 / 0280945A1).
[0206] Chimeric antibodies against MAGE-A4 were initially isolated with human variable regions and mouse constant regions. Antibodies are characterized and selected for desired characteristics including affinity, selectivity, etc. If desired, fully human anti-MAGE-A4 antibodies are produced by replacing th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com