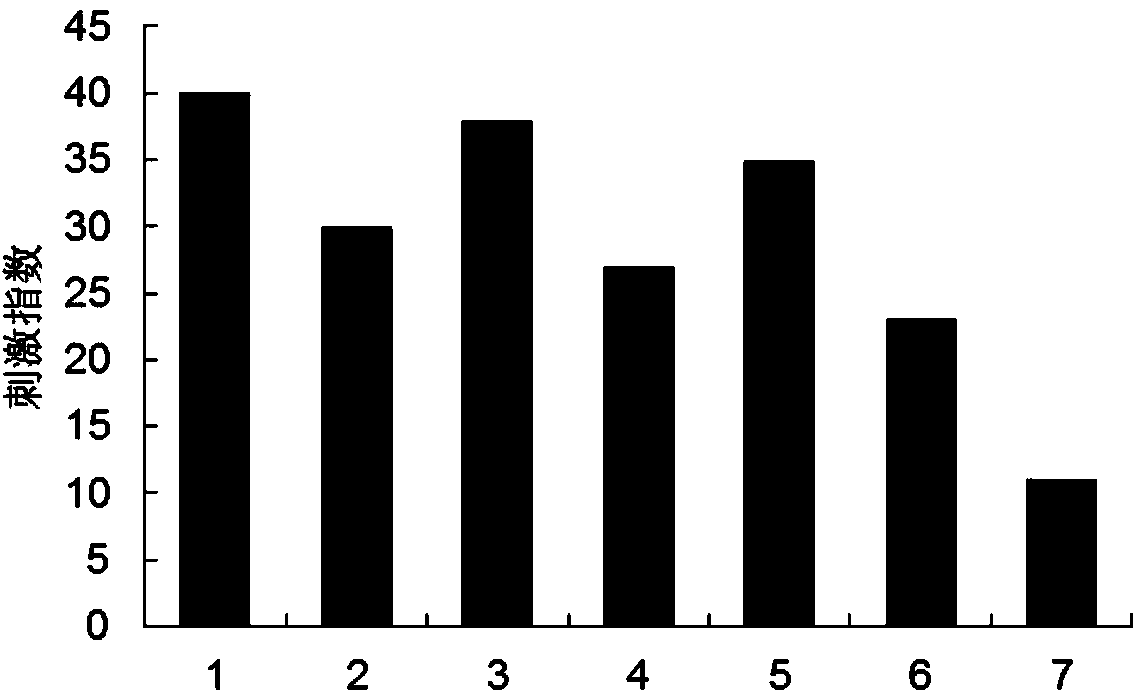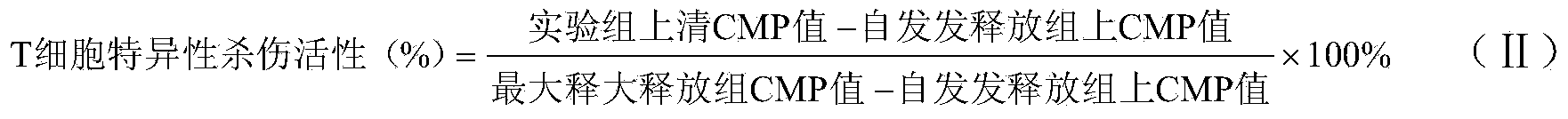Malignant melanoma resisting vaccine composition and application thereof
A technology of malignant melanoma and vaccine composition, applied in the field of biopharmaceuticals, can solve the problem of increasing the specific anti-tumor immune response of malignant melanoma vaccine, inability to effectively induce or activate the patient's immune response, malignant melanoma specificity and immune response Weak response and other problems, to achieve strong specific killing function, good therapeutic effect, and easy to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
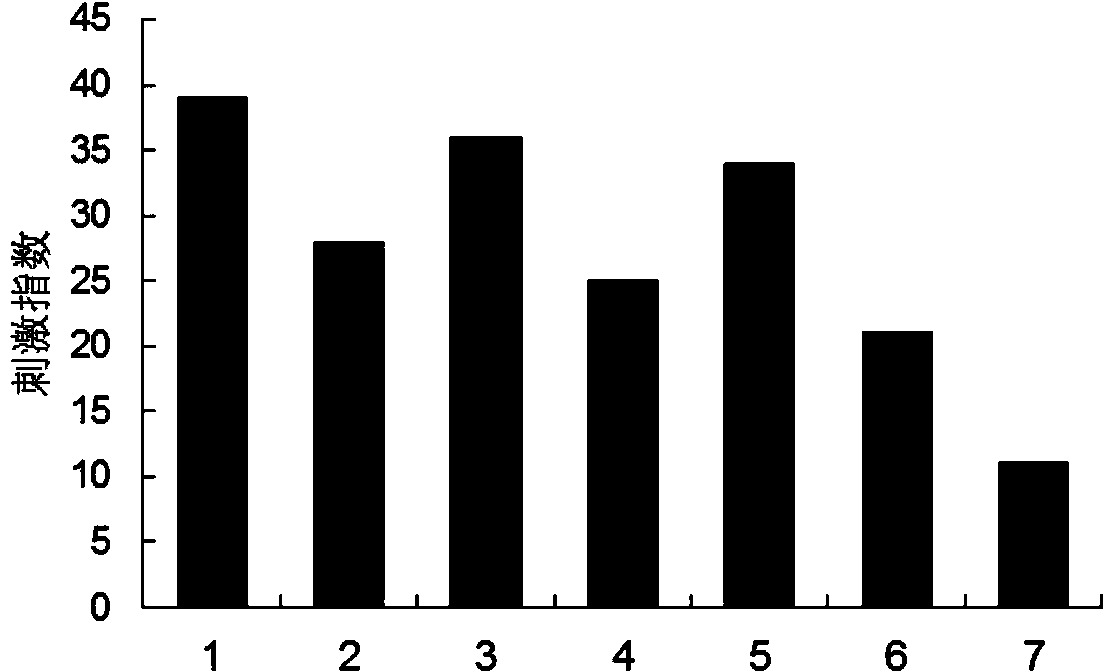
Image
Examples
Embodiment 1
[0040] 1. Preparation of malignant melanoma antigen
[0041] (1) Preparation of malignant melanoma attenuated cells
[0042] M3 malignant melanoma cell line (H-2 d )(2xl0 6 / mL) into a 6-well plate, and then 60 Co irradiation 30Gy spare.
[0043] (2) Preparation of DNP-modified malignant melanoma attenuated cells
[0044] M3 malignant melanoma cell line (H-2 d )(2xl0 6 / mL) into a 6-well plate, and then 100L of DNP solution to a final concentration of 0.2% (5mmol / L, pH7.2), incubated at 37°C for 10min, and then 60 Co irradiation 30Gy spare.
[0045] 2. Preparation of antigen mixture containing tuberculosis antigen, malignant melanoma antigen and rod-shaped bodies
[0046] Take the malignant melanoma antigen according to the amount of 80%-100% (volume), take the tuberculosis antigen according to the amount of 0%-20% (volume), and take the rod-shaped bodies according to the amount of 0%-20% (volume) and mix them evenly Backup.
[0047] 3. Preparation and activation of DC...
Embodiment 2
[0088] 1. Preparation of malignant melanoma antigen
[0089] (1) Preparation of malignant melanoma attenuated cells
[0090] The logarithmic growth of malignant melanoma cell line B16 (H-2 b )(1xl0 7 / mL) into a 6-well plate, and then 60 Co irradiation 30Gy spare.
[0091] (2) Preparation of DNP-modified malignant melanoma attenuated cells
[0092] The logarithmic growth of malignant melanoma cell line B16 (H-2 b )(1xl0 7 / mL) into a 6-well plate, and then 100 μL of DNP solution to a final concentration of 0.2% (5mmol / L, pH7.2), incubated at 37°C for 10min, and then 60 Co irradiation 30Gy standby, dose rate 1.88Gy / min.
[0093] 2. Preparation of antigen mixture containing tuberculosis antigen, malignant melanoma antigen and rod-shaped bodies
[0094] Take malignant melanoma antigen B16 attenuated cells according to the amount of 80%-98% (volume), take tuberculosis antigen BCG according to the amount of 2%-10% (volume), and take rod-shaped small Mix well and set aside. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com