T cell receptors recognizing hla-a1- or hla-cw7-restricted mage
a technology of t-cell receptors and mage, which is applied in the direction of immunoglobulins, peptides, drugs, etc., can solve the problems that patients without hla-a2 expression cannot be treated with t-cells, and hinder the widespread application of adoptive cell therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0115]This example demonstrates the cloning of TCR genes from T cell clones and the generation of TCR constructs.
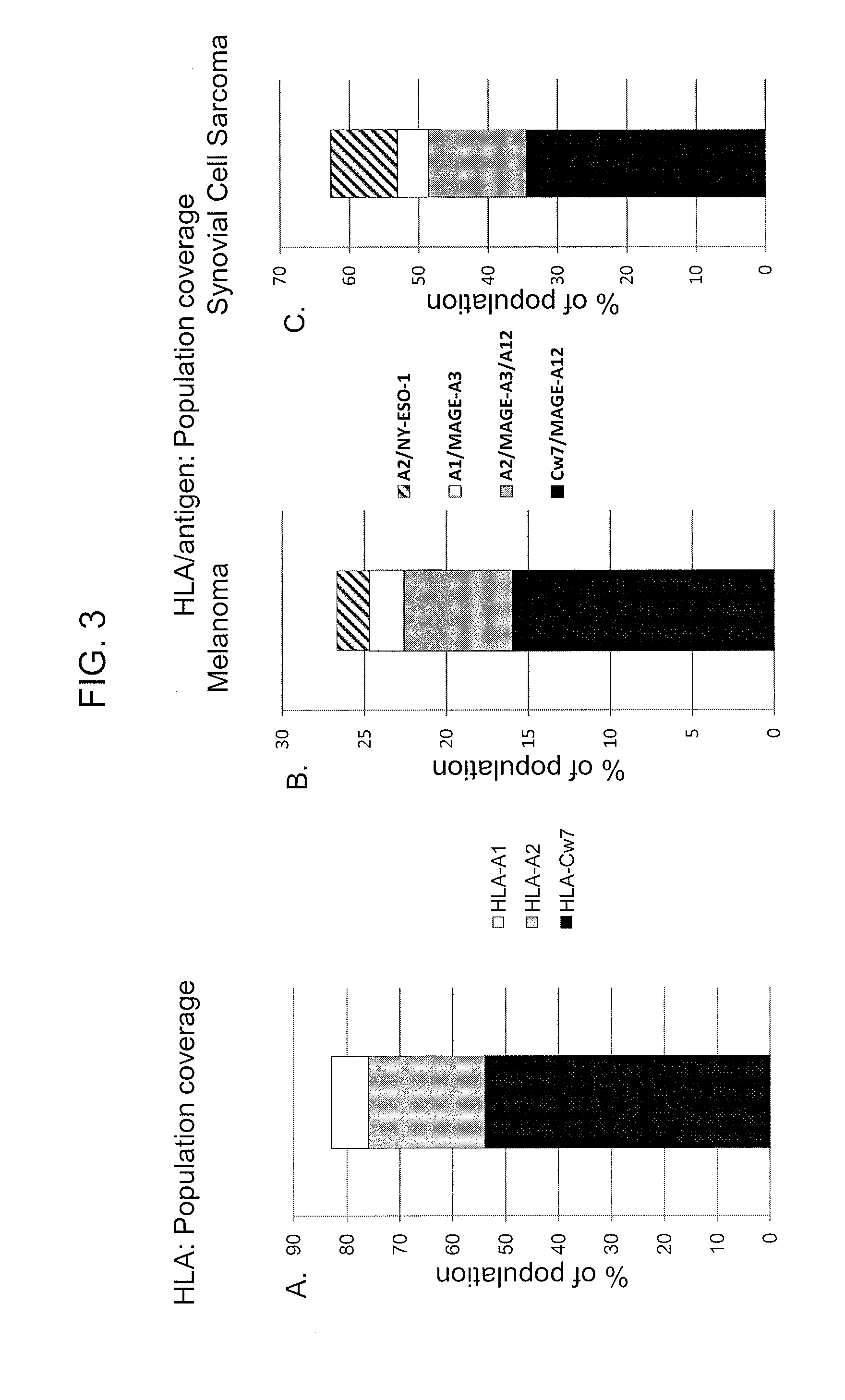
[0116]Four T cell clones were initially identified that recognized epitopes of the MAGE-A gene family in the context of the dominant class I alleles HLA-A*01 and C*07. Approximately 30% of the melanoma patient population expresses HLA-A*01, and more than 95% of HLA-A*01+ individuals express the HLA-A*0101 sub-type, while more than 50% of melanoma patients express one of the two dominant HLA-C*07 sub-types, C*07:01 and C* 07:02.
[0117]The expressed TCR α and β chains were isolated from two clones, A10 and 13-18, that recognized residues 168-176 of protein MAGE-A3 (MAGE-A3:168-176) in the context of HLA-A*01. In addition, HLA-C*07 restricted TCRs recognizing a peptide corresponding to residues 170-178 of the MAGE-A12 protein (MAGE-A12:170-178) were isolated from clones 502 and FM8.
[0118]The α and β chains encoding functional TCRs were isolated from two MAGE-A12 reactive, HLA...
example 2
[0120]This example demonstrates the reactivity of cells expressing anti-MAGE-A3 TCR-A10 (SEQ ID NOs: 13 and 14) and anti-MAGE-A3 TCR 13-18 (SEQ ID NOs: 24 and 25) in response to HLA-A1+ / MAGE-A3+ cells.
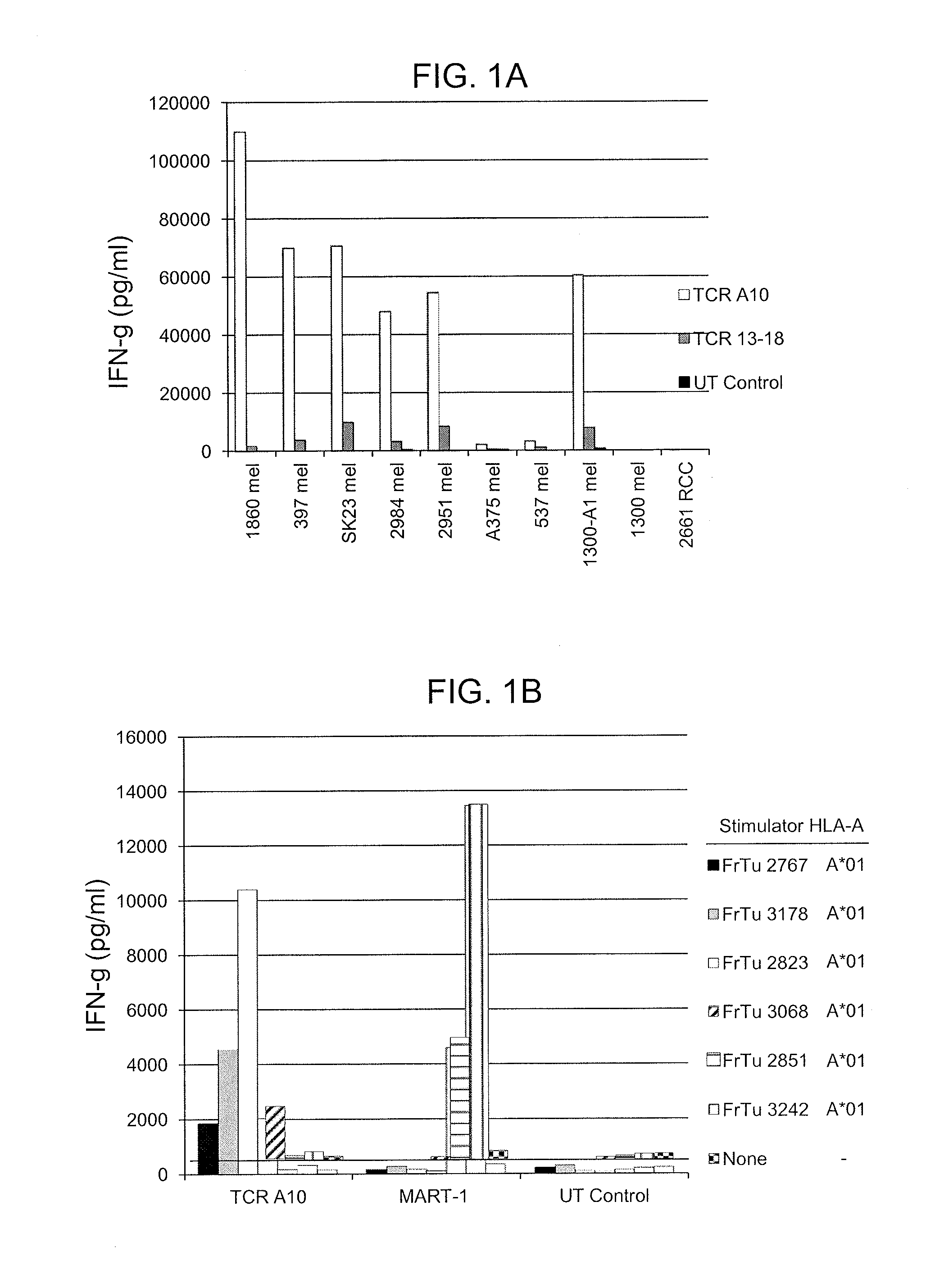
[0121]Anti-CD3 stimulated T cells transduced with TCR-A10 (SEQ ID NO: 46) and TCR 13-18 (SEQ ID NO: 48) were evaluated for their ability to recognize a panel of HLA-A*01+melanoma cell lines that express MAGE-A3. Untransduced (UT) and transduced cells were co-cultured overnight with various tumor cell lines (Tables 1A, 1B and FIG. 6A), and interferon-gamma (IFN-γ) (pg / ml) was measured.
TABLE 1ATumorHLA-A*01Copies MAGE-A31860 mel+12,100397 mel+32,700SK23 mel+18,4002984 mel+14,9002951 mel+12,300A375 mel+3,670537 mel+4,2701300-A1 mel+7,2801300 mel—13,6002661 RCC+
TABLE 1BTumorHLA-A*01MAGE-A32984 mel++397 mel++2630 mel++2556 mel++526 mel—+624 mel—+2359 mel—+2661 RCC+—
[0122]The results indicate that six of the eight HLA-A*01+ / MAGE-A3+ melanoma cell lines that were evaluated stimulated higher l...
example 3
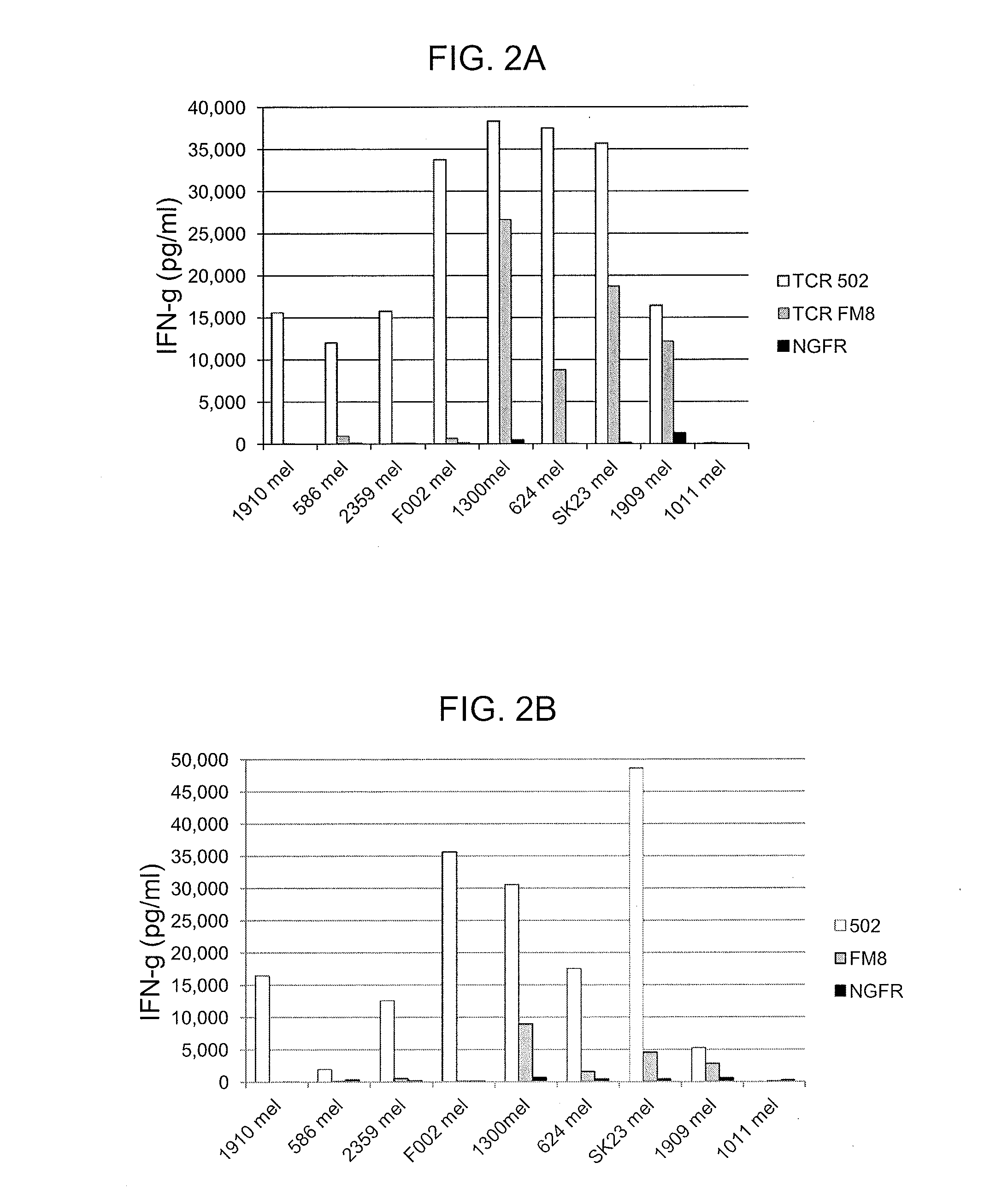
[0129]This example demonstrates the reactivity of cells expressing anti-MAGE-A12 TCR 502 (SEQ ID NOs: 34 and 35) or anti-MAGE-A12 TCR FM8 (SEQ ID NOs: 44 and 45) in response to co-culture with HLA-Cw*07+ / MAGE-A12+ cells.
[0130]Anti-CD3 stimulated CD8+ T cells isolated from two patient PBMC samples were transduced with a control construct encoding the truncated human low affinity nerve growth factor receptor (NGFR), TCR 502 (SEQ ID NO: 47), or TCR FM8 (SEQ ID NO: 49) were evaluated for their ability to recognize a panel of Cw*07+ melanoma cell lines that express MAGE-A 12.
[0131]Expression of the MAGE-A12 gene product was evaluated by Q-PCR using two primers (SEQ ID NOs: 61 and 62) designed to specifically amplify the MAGE-A12 gene product but not other members of the MAGE-A gene family as well as a MAGE-A12 specific probe (SEQ ID NO: 63). Antigen expression was determined using plasmid controls as standards for estimating copy numbers and using glyceraldehyde 3-phosphate dehydrogenase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com