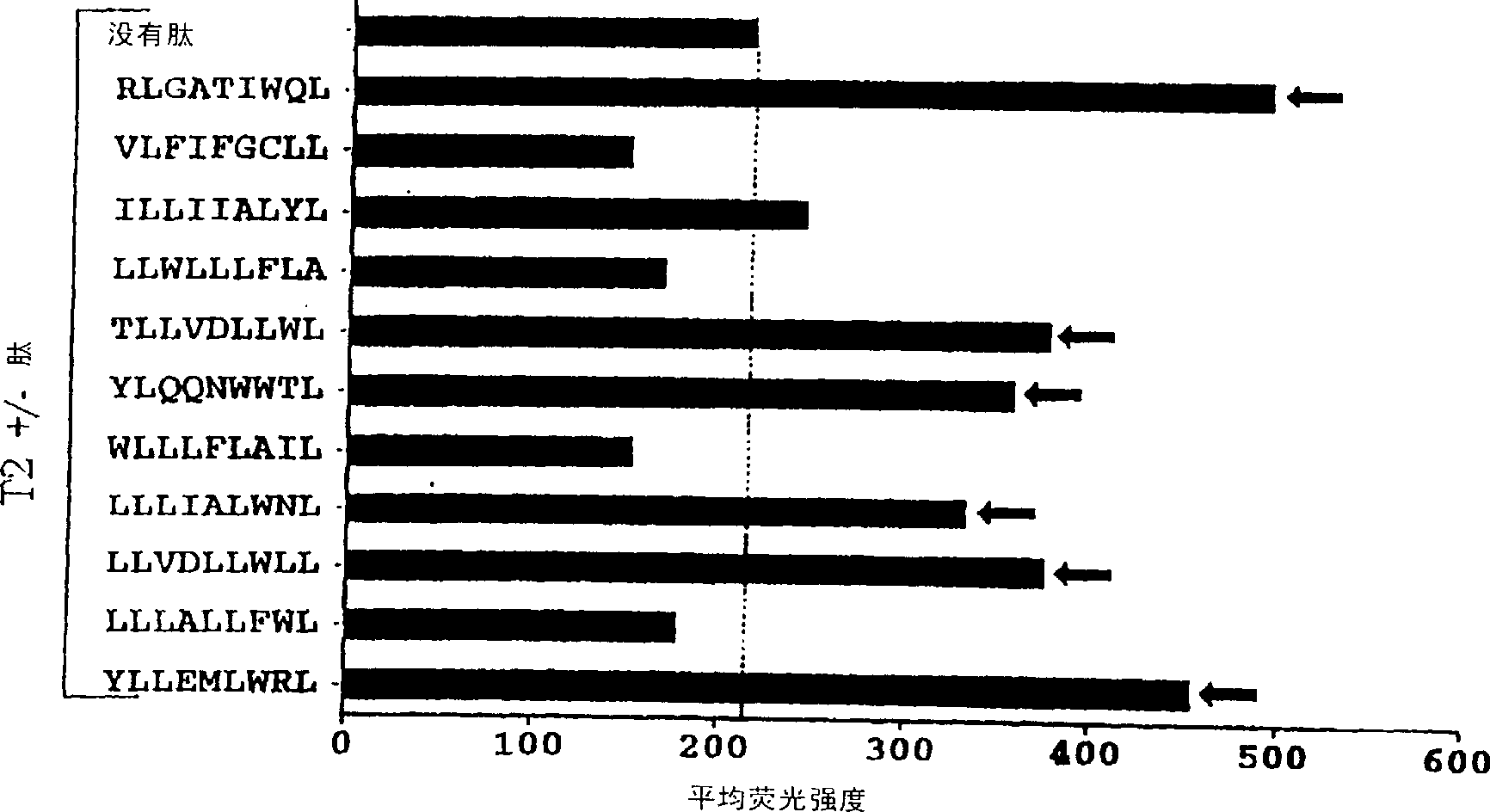
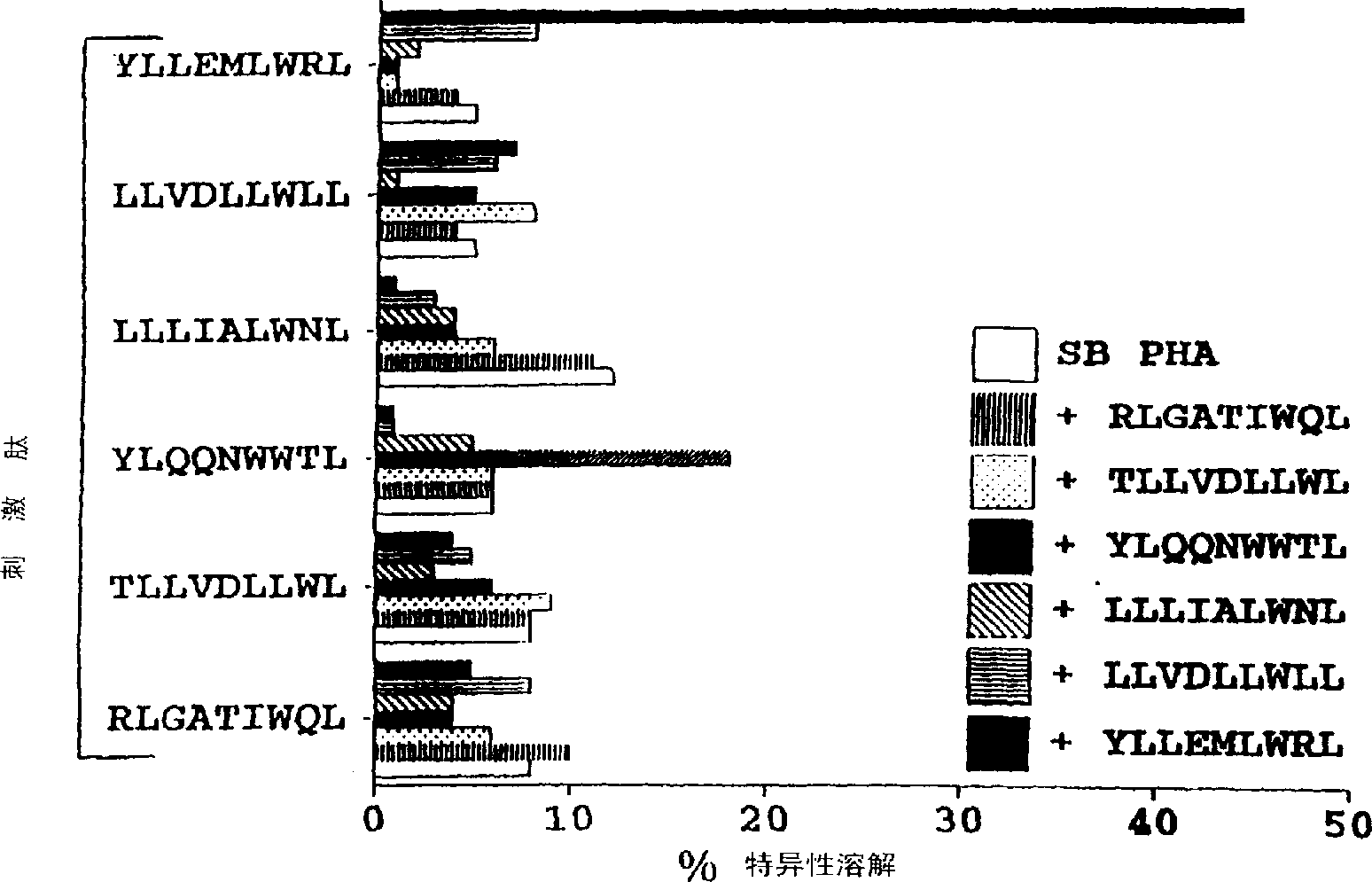
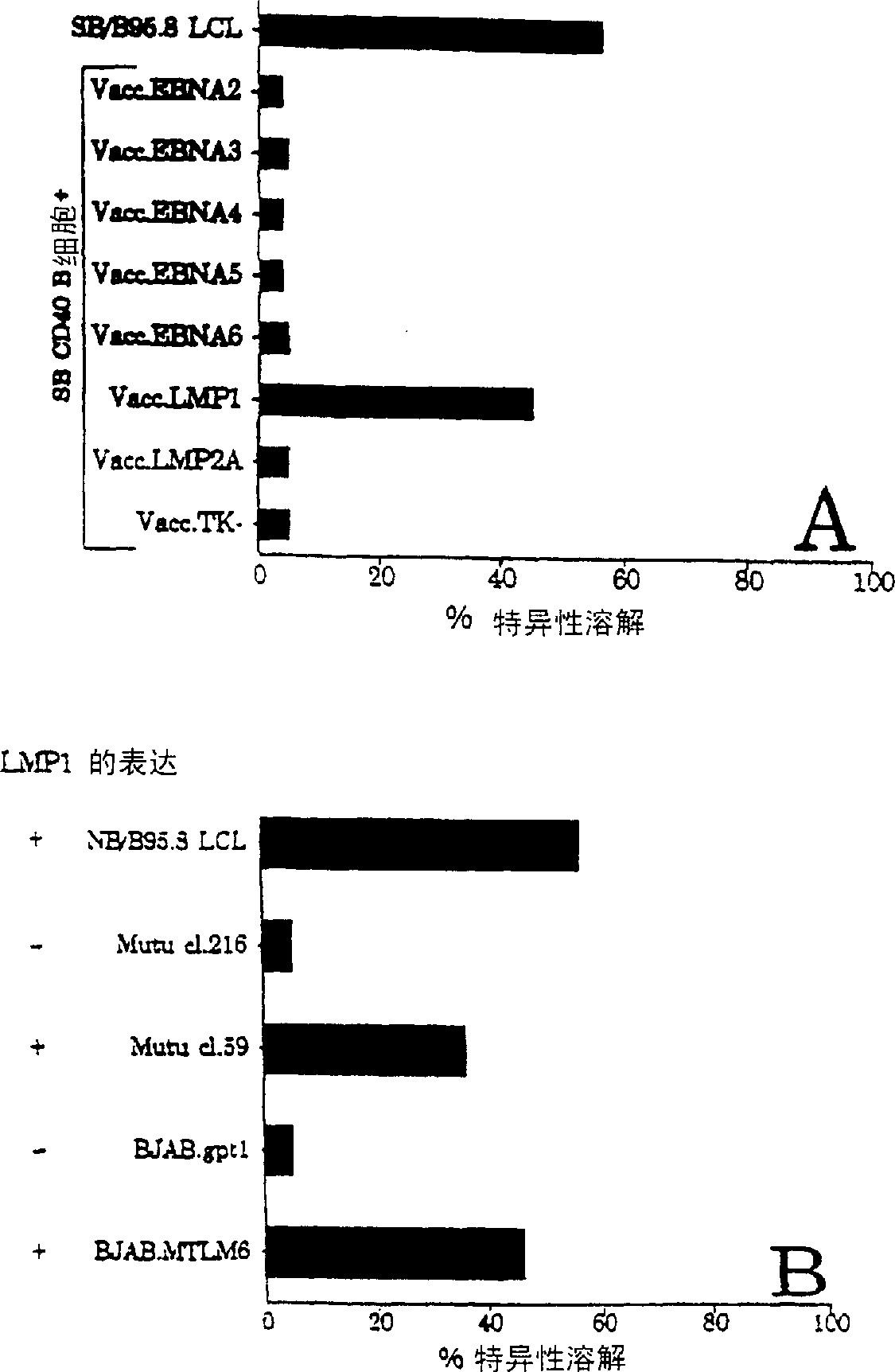
CTL epitopes from EBV
An epitope-free technology, applied in the field of subunit vaccines and nucleic acid vaccines, can solve problems such as blocking CTL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Materials and methods
[0072] Cell line establishment and maintenance
[0073] LCLs were established from seropositive donors by heterologous viral transformation of peripheral B cells with B95.8 (type 1) or Ag876 (type 2) virus isolates. Furthermore, LCLs transformed with the B95.8 isolate expressing different HLA A2 supertypes (12th Histocompatibility Symposium cell series; EACC) were also used in this study. The peptide transporter (TAP) negative BxT hybrid cell line 174xCEM.T2 (denoted T2) (22) was used for peptide stability assays. All cell lines were routinely cultured in RPMI 1640 containing 2 mM glutamate, 100 IU / ml penicillin, 100 μg / ml streptomycin plus 10% fetal calf serum (FCS) (growth medium). Long-term cultures of EBV-negative normal B-cell blasts were established using the CD40 system (represented by CD40 B cells) as previously described (12).
[0074] The Burkitt lymphoma (BL) cell lines BJAB.gptl, BJAB.MTLM6, MUTUcl.59 and MUTUcl.216 were used in th...
Embodiment 2
[0118] Materials and methods
[0119] Patients with infectious mononucleosis (IM)
[0120] IM patients clinically identified as positive for metachromatic antibodies were bled 5-10 days after the onset of illness. In two cases, these patients were HLA-typed for the HLA A2 allele with microcytotoxic serotyping and genotyping 24-36 months after the end of the second phase of symptoms. Three patients (SB, LP, and MG) were identified as HLAA2-positive, which was subsequently confirmed by FACS analysis with an HLA A2-specific monoclonal antibody (ATCC).
[0121] Cell line establishment and maintenance
[0122] Transformation of peripheral B cells with type 1 (B95.5) or type 2 (Ag876) EBV isolates via exogenous virus, EBV-transformed lymphoblastoid cell lines (LCLs) from IM and healthy EBV seropositive donors Cell plates were established (16) and routinely maintained in RPMI 1640 (growth medium) containing 2 mM glutamate, 100 IU / ml penicillin and 100 μg / ml streptomycin plus 10% f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com