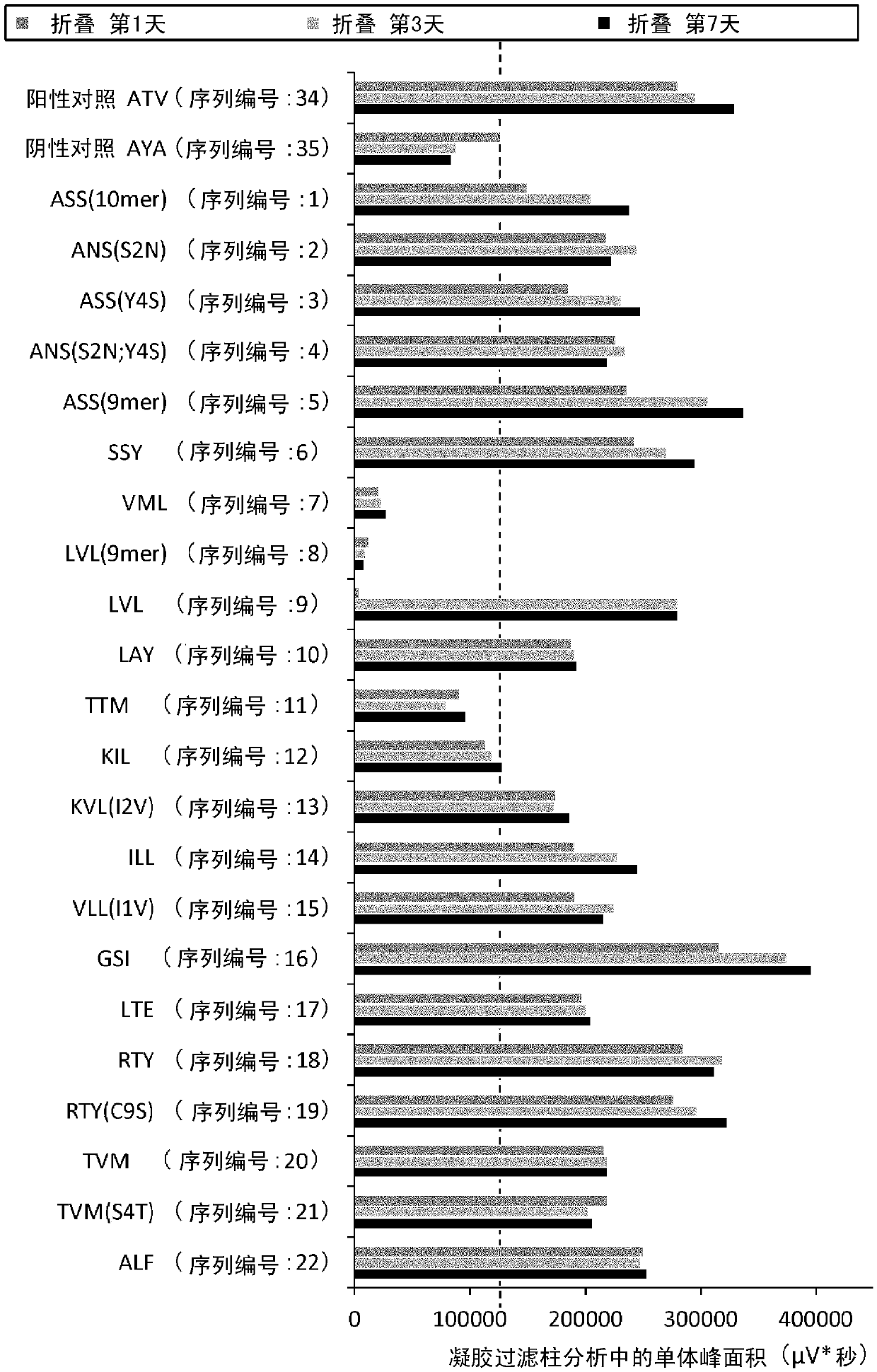
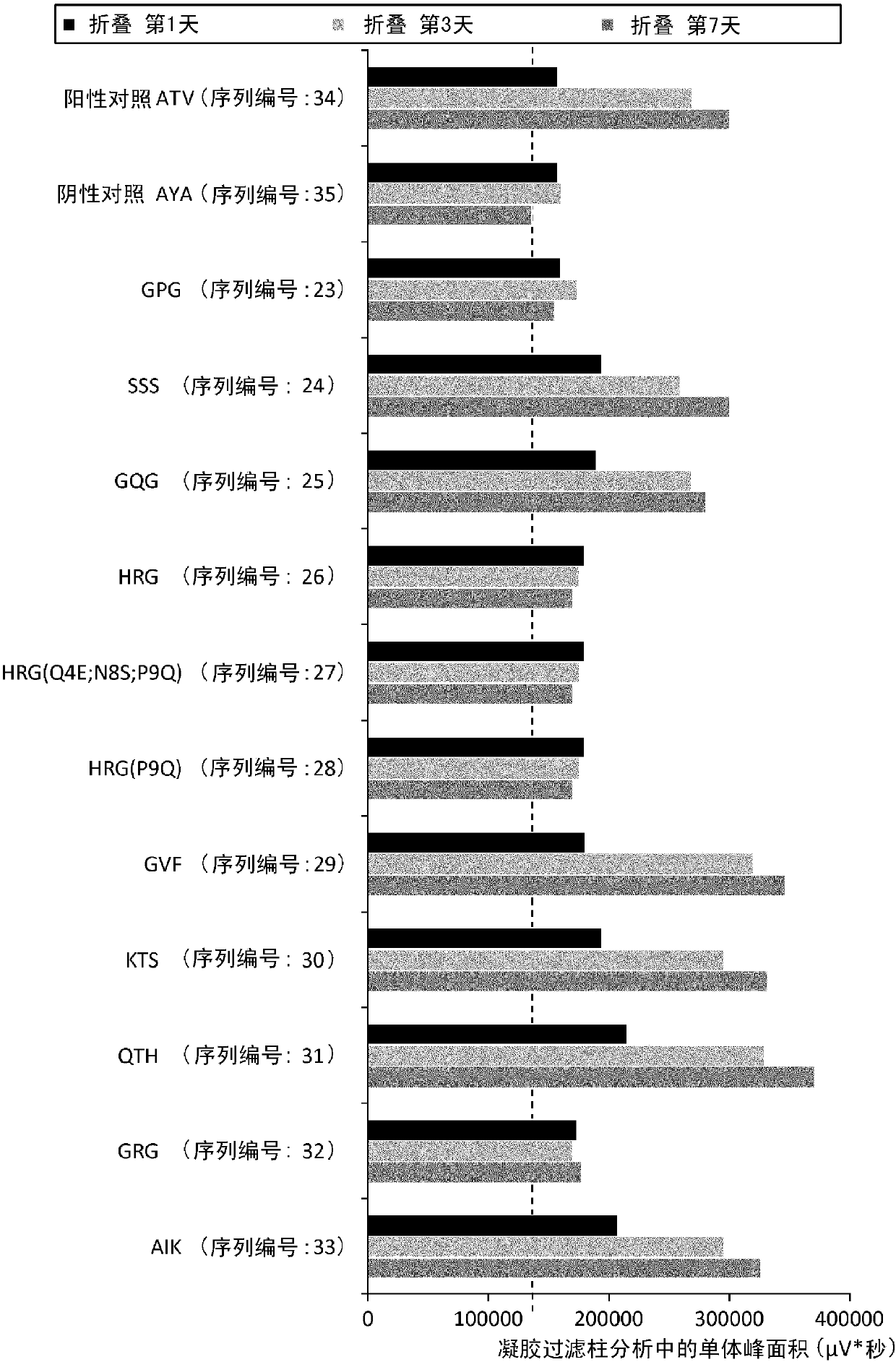
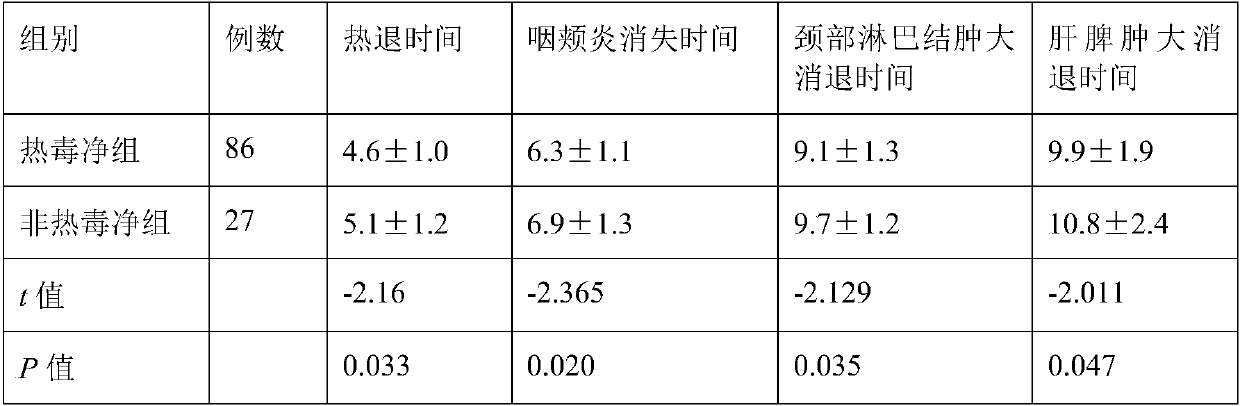
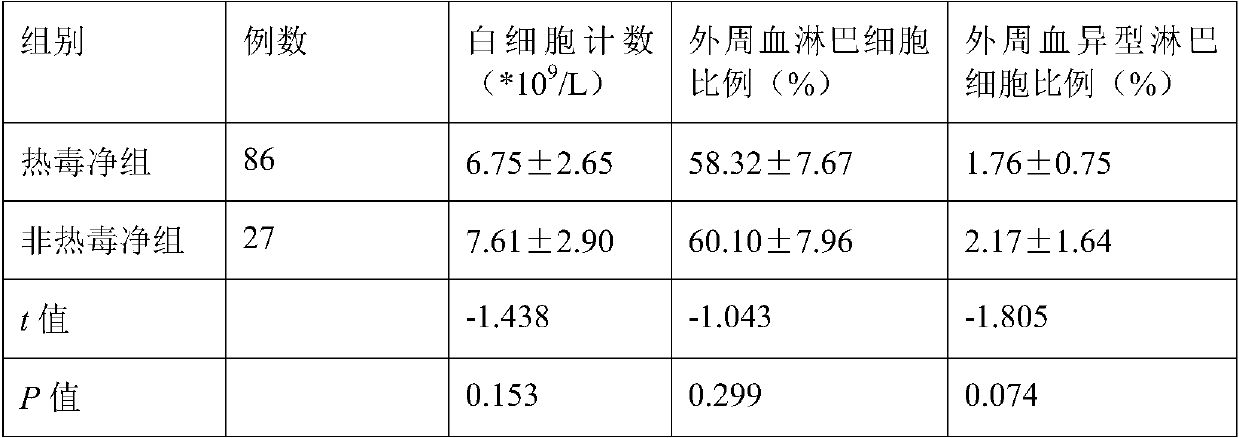
Patents
Literature
31 results about "Ebv infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for the inhibition of epstein-barr virus transmission employing anti-viral peptides capable of abrogating viral fusion and transmission
InactiveUS6518013B1SsRNA viruses negative-sensePeptide/protein ingredientsCell membraneViral life cycle
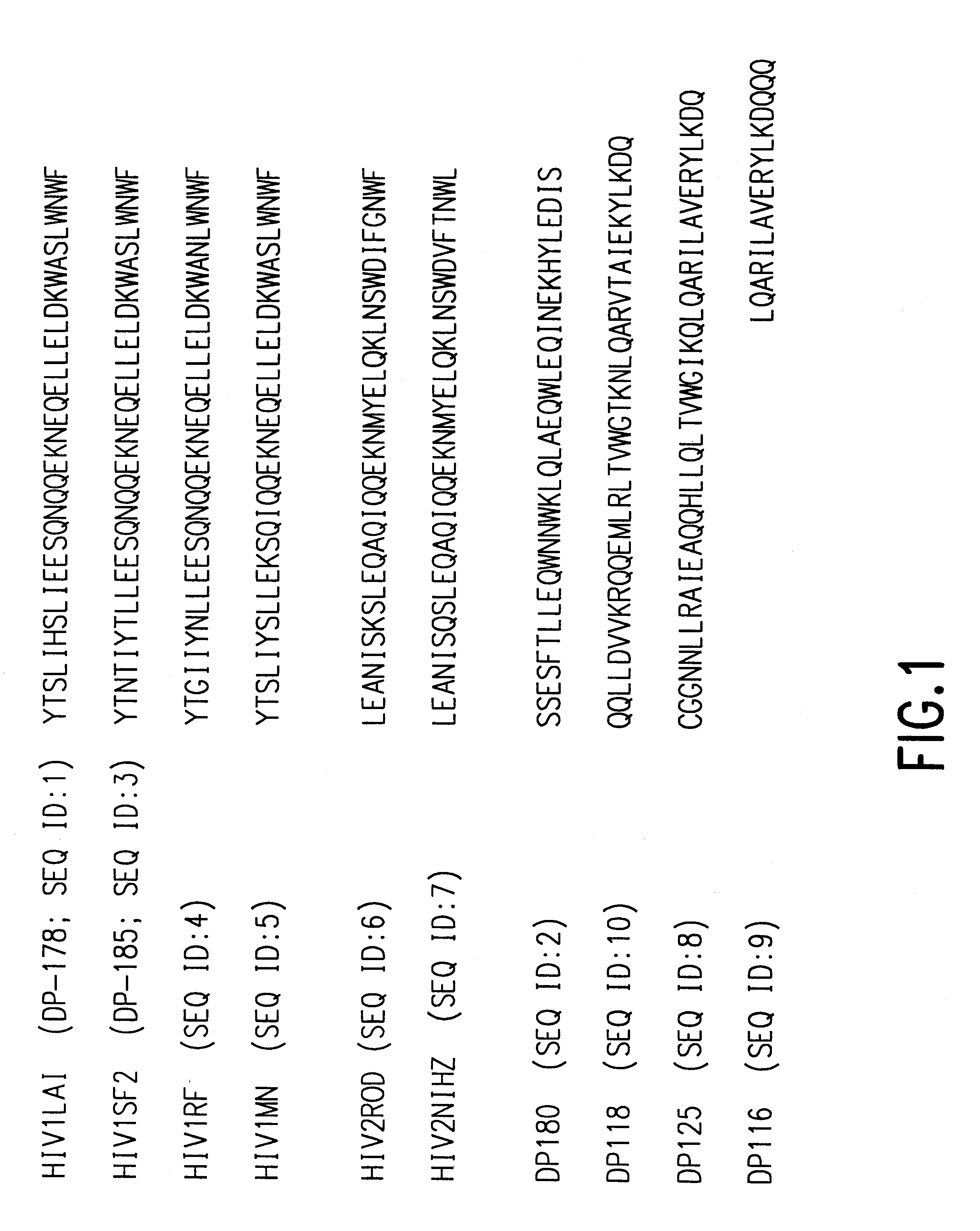
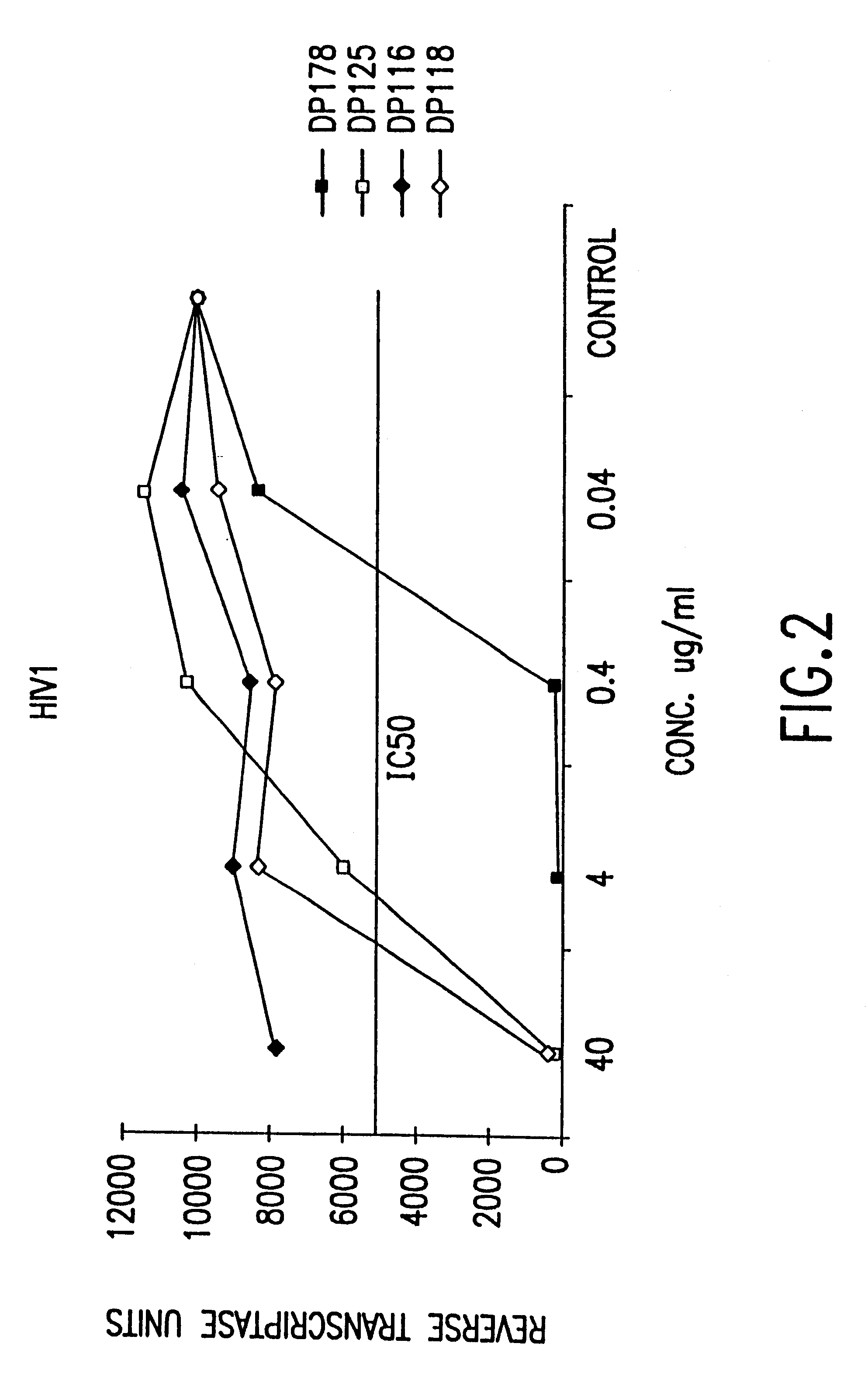
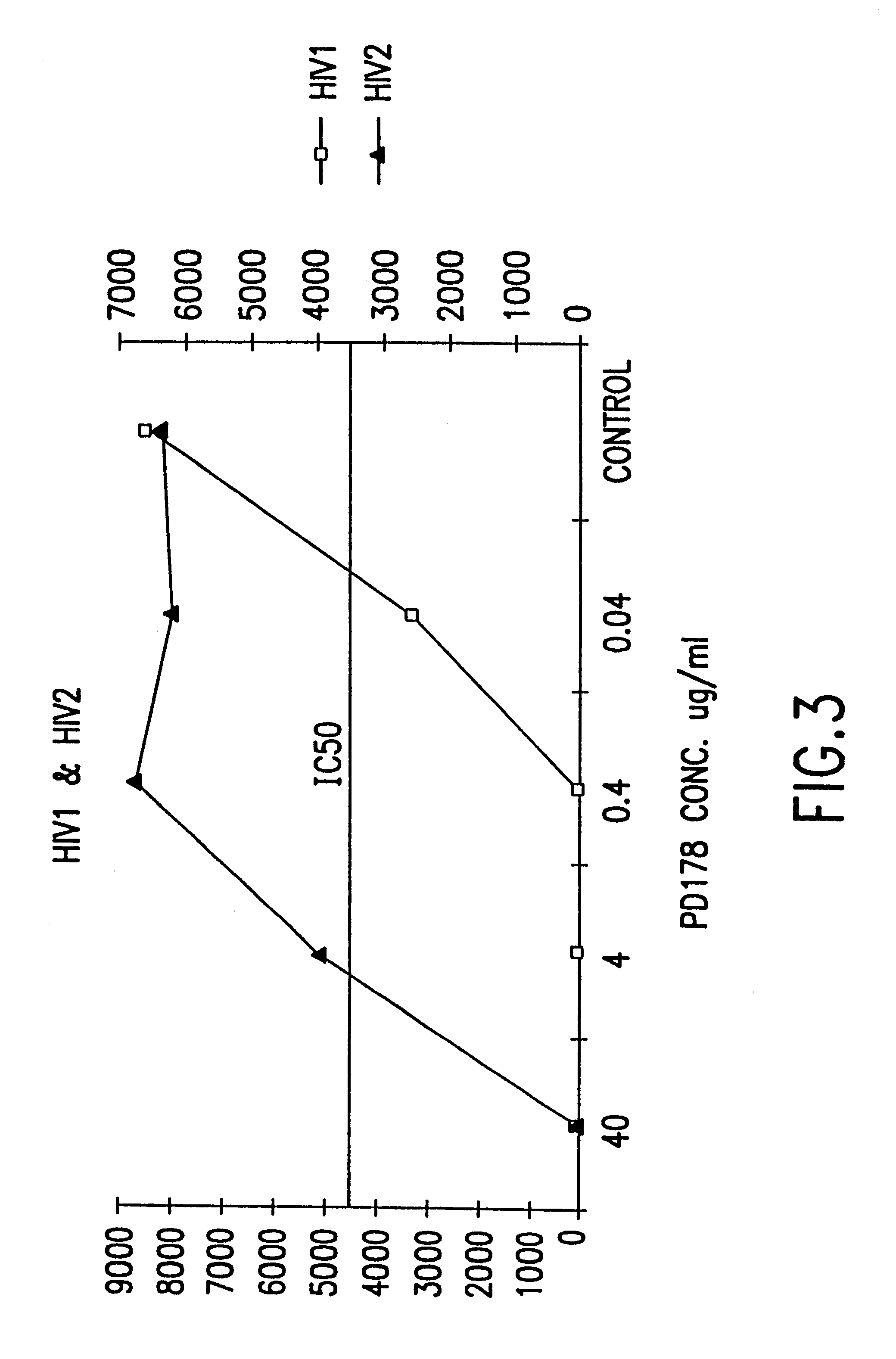
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1(HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI 5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the Epstein-Barr virus (EBV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the EBV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of EBV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of EBV infections.
Owner:TRIMERIS
CTL epitopes from EBV
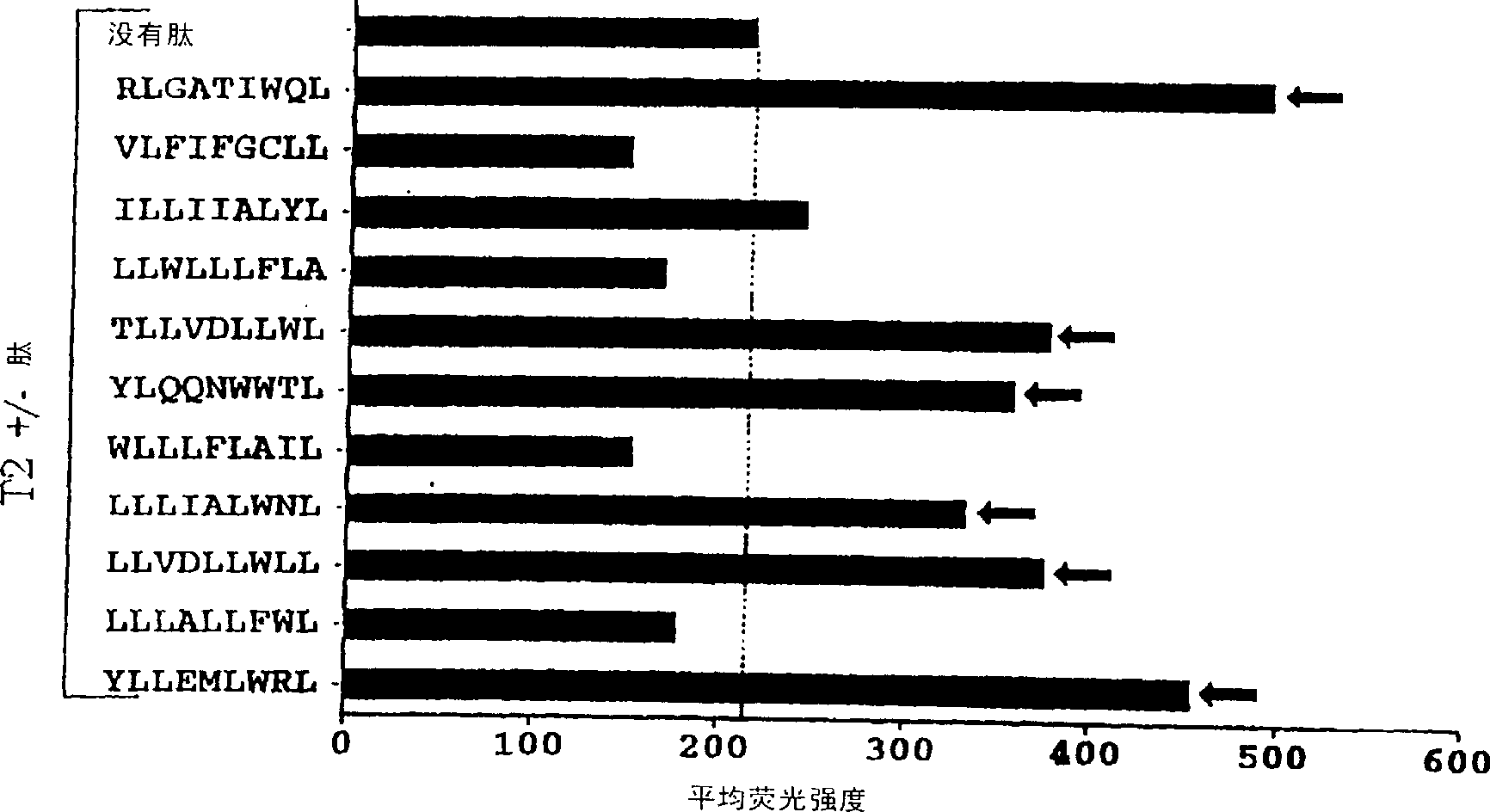
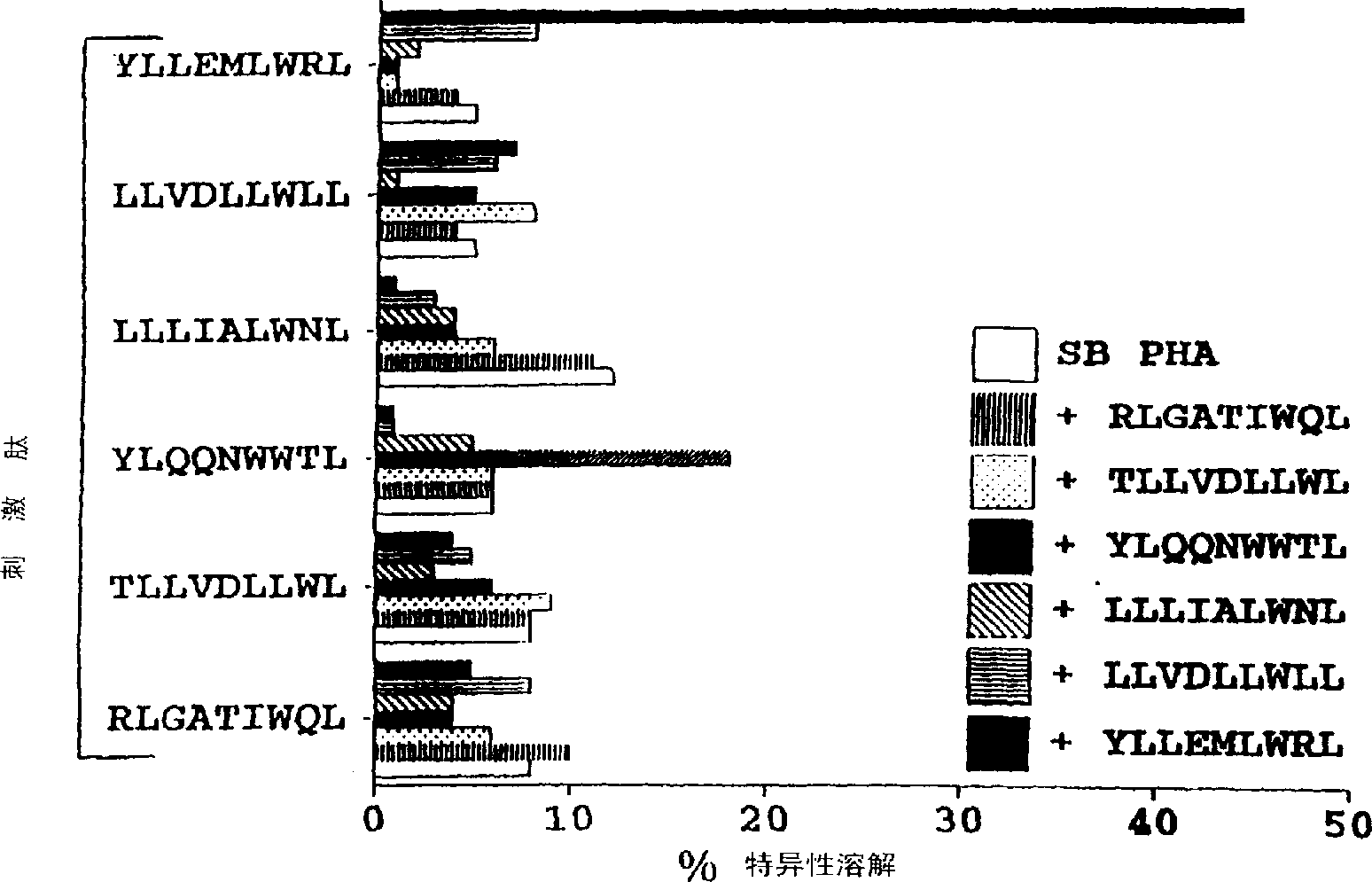
The present invention provides cytotoxic Epstein-Barr virus (EBV) T-cell epitopes derived from EBV structural antigens. Preferred epitopes include YLLEMLWRL (SEQ ID NO:1), YFLEILWGL (SEQ ID NO:32), YLLEILWRL (SEQ ID NO:33), YLQQNWWTL (SEQ ID NO:6), LLLALLFWL (SEQ ID NO:2), LLVDLLWLL (SEQ ID NO:3), LLLIALWNL (SEQ ID NO:4), WLLLFLAIL (SEQ ID NO:5), TLLVDLLWL (SEQ ID NO:7), LLWLLLFLA (SEQ ID NO:8), ILLIIALYL (SEQ ID NO:9), VLFIFGCLL (SEQ ID NO:10), RLGATIWQL (SEQ ID NO:11), ILYFIAFAL (SEQ ID NO:15), SLVIVTTFV (SEQ ID NO:17), LMIIPLINV (SEQ ID NO:20), TLFIGSHVV (SEQ ID NO:24), LIPETVPYI (SEQ ID NO:26), VLQWASLAV (SEQ ID NO:27) and QLTPHTKAV (SEQ ID NO:29). The present invention also provides methods of treating or preventing EBV infection in subjects which involve administration of EBV cytotoxic T-cell epitopes.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Protective antigen of epstein barr virus
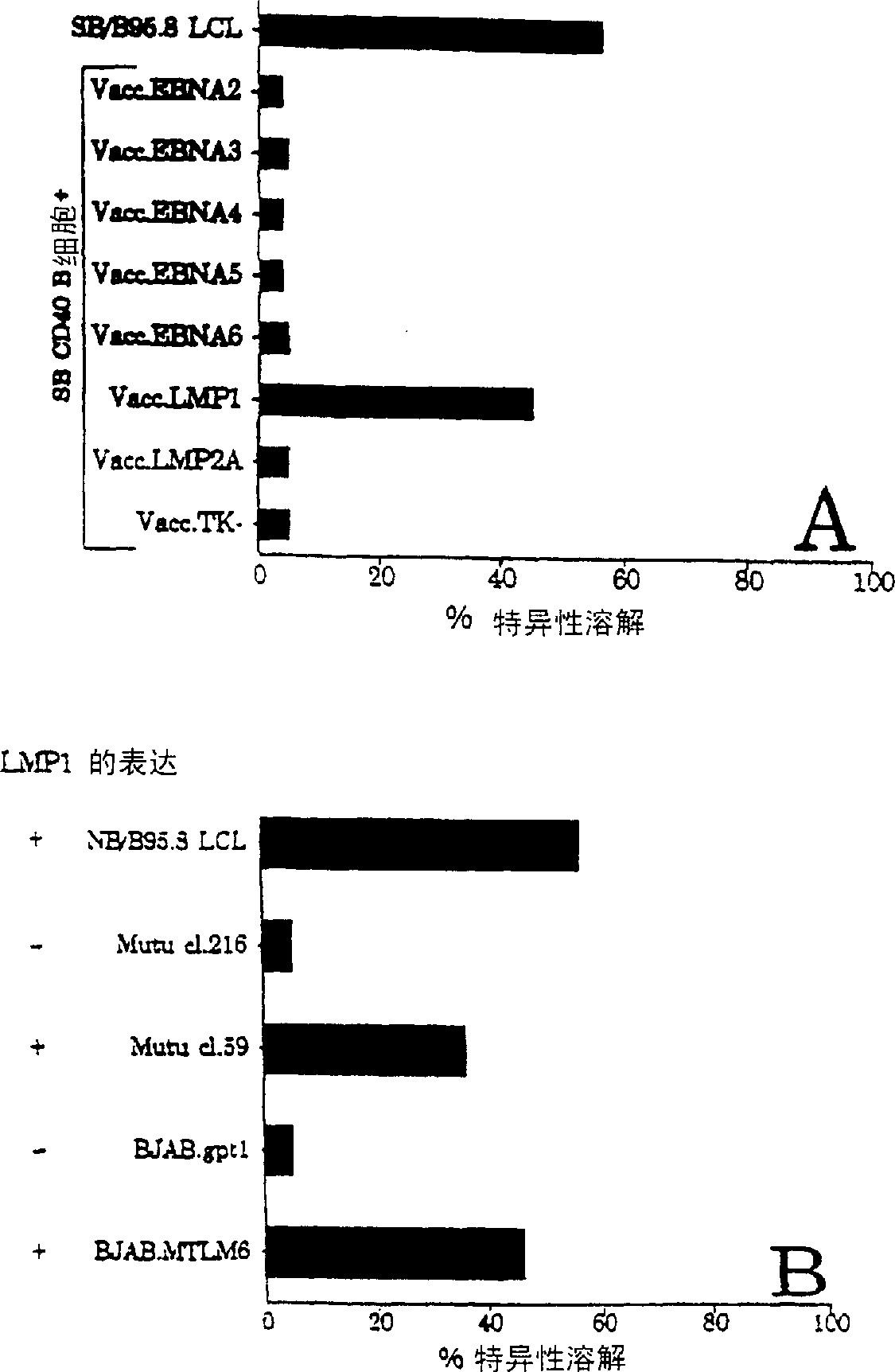
The present invention relates to the identification of a subunit vaccine to prevent or treat infection of Epstein Barr Virus. In particular, EBNA-1 was identified as a vaccine antigen. In a specific embodiment, a purified protein corresponding to EBNA-1 elicited a strong CD4+ T cell response. The responsive CD4+ T cell are primarily TH1 in function. EBNA-1 is an attractive candidate for a protective vaccine against EBV, and for immunotherapy of EBV infection and neoplasms, particularly with dendritic cells charged with EBNA-1.
Owner:THE ROCKEFELLER UNIV
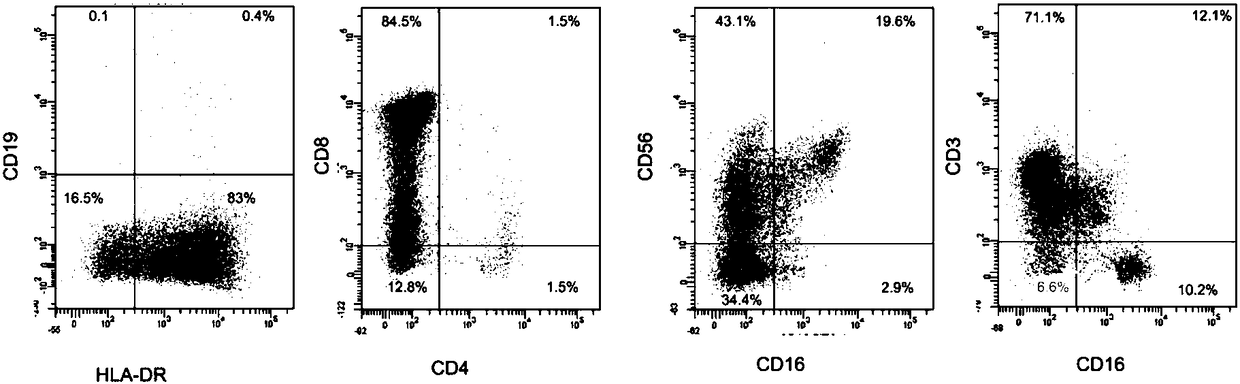
Identification method of EB virus infected lymphocyte subpopulation and application thereof
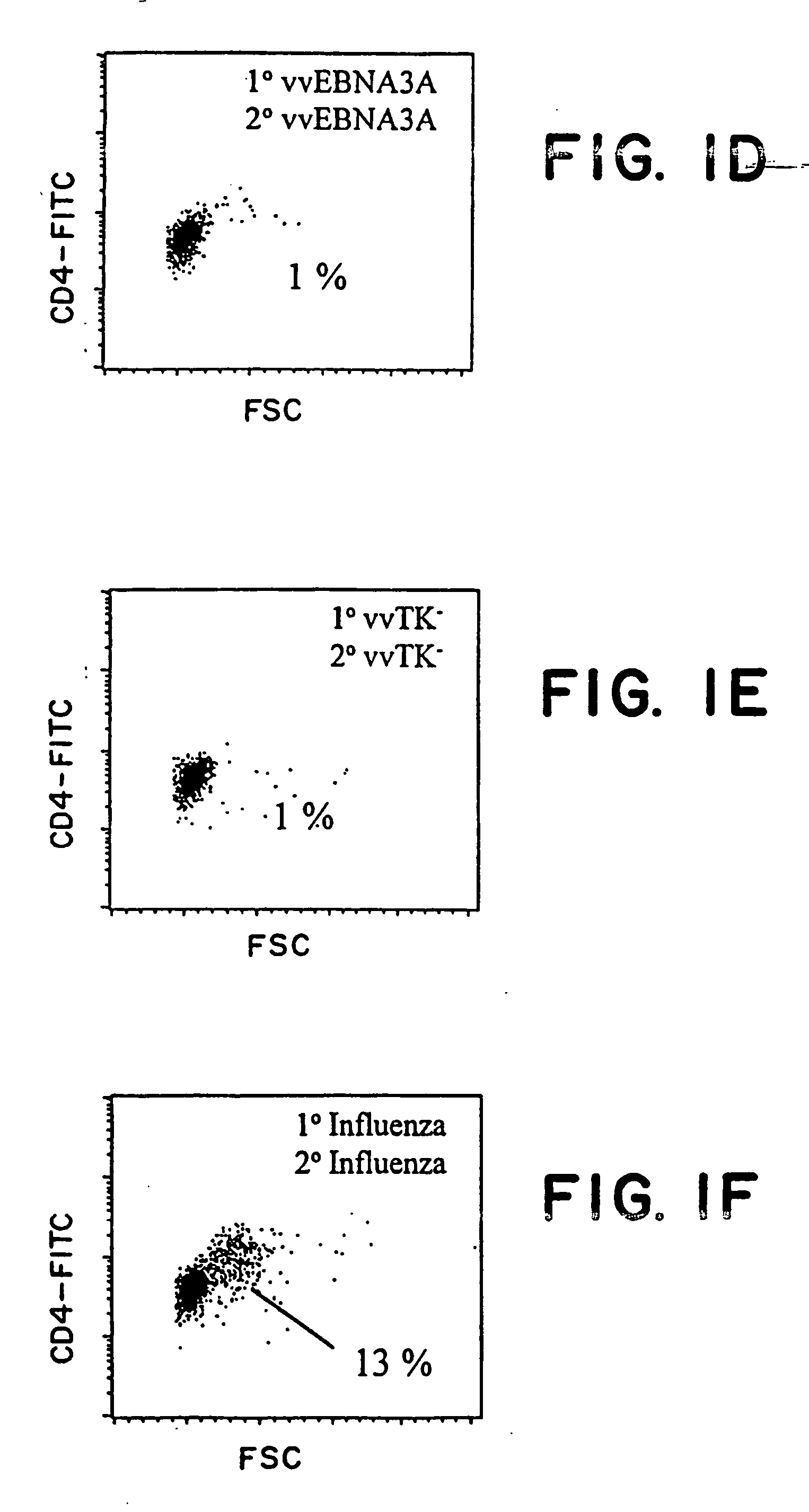
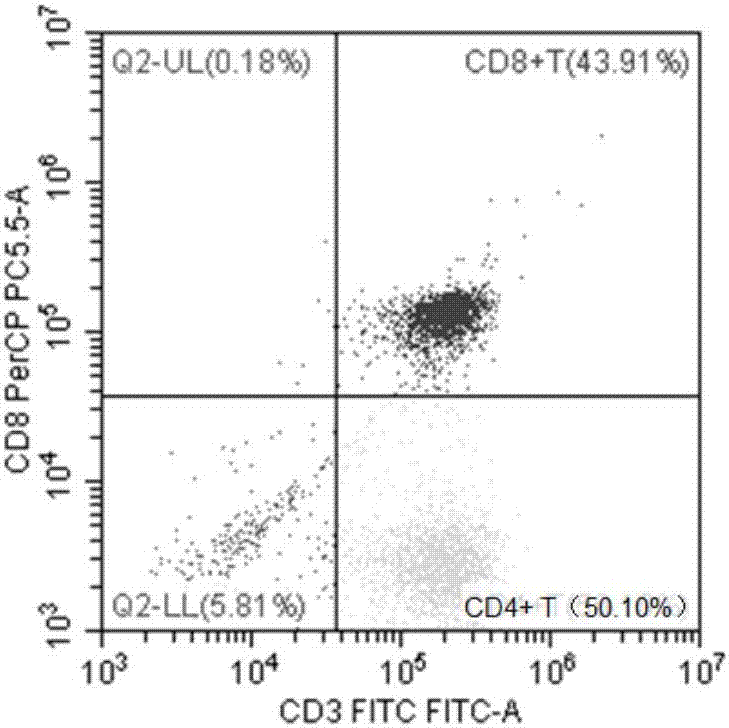
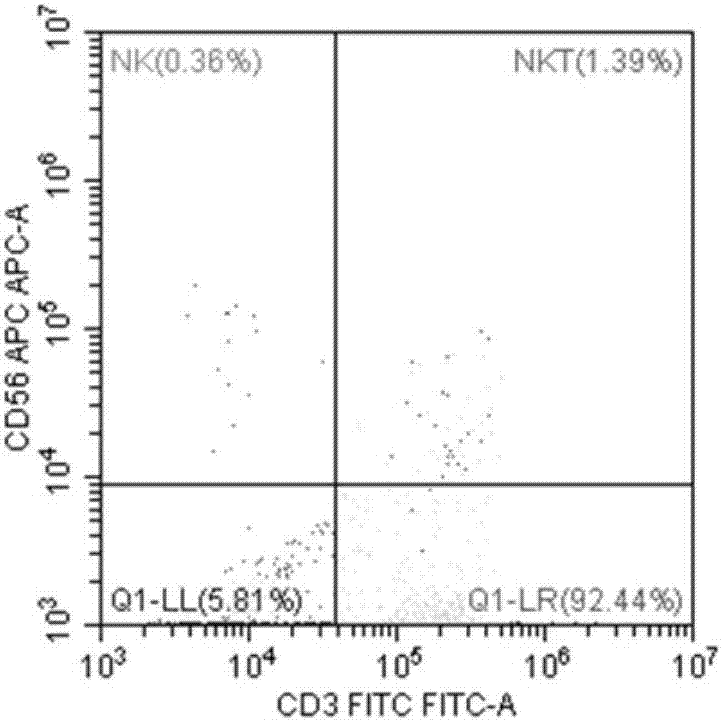
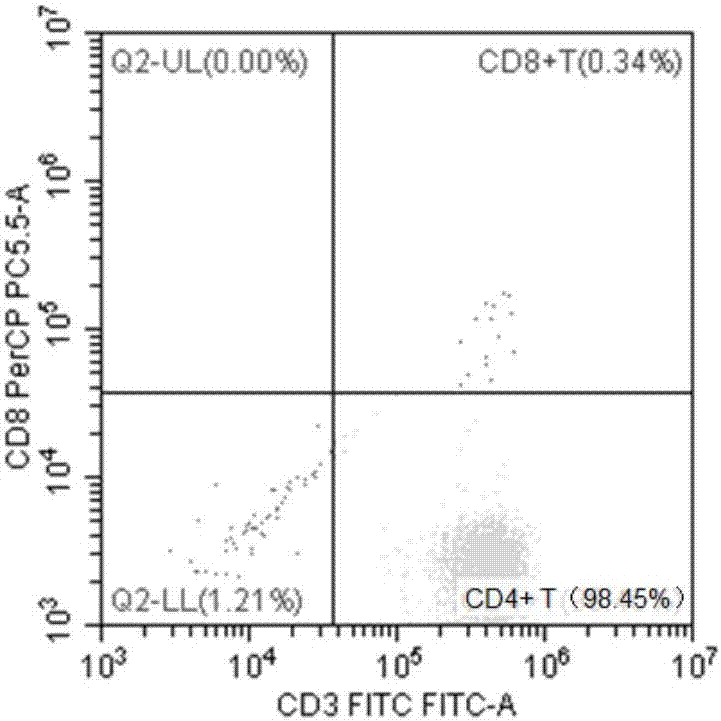
ActiveCN106947835APrecise positioningEffective treatmentMicrobiological testing/measurementMicroorganism based processesEbv infectionMonocyte
The invention provides an identification method of an EB virus infected lymphocyte subpopulation and an application thereof and relates to the technical field of medical detection. The identification method of the EB virus infected lymphocyte subpopulation provided by the invention can accurately position the cell type of EBV infection by sorting monocytes of peripheral blood of an EBV infected patient in combination with a flow cytometry and a real-time fluorescent quantitative PCR technology, and is helpful for a clinician to formulate a therapeutic regimen for the cell type with EBV infection so as to efficiently and accurately treat diseases caused by EBV infection. In addition, by applying the identification method provided by the invention, target cells infected with EBV can be determined, preparation of related therapeutic drugs can be guided, and the targeted drugs are prepared with a clear target.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
Molecular target for diagnosing and treating nasopharyngeal cancer related with Epstein-Barr virus (EBV) infection and application thereof
InactiveCN102174516AAccurately reflect retention and lossPrevent proliferationMicrobiological testing/measurementGenetic material ingredientsEBV InfectionsNasopharyngeal carcinoma
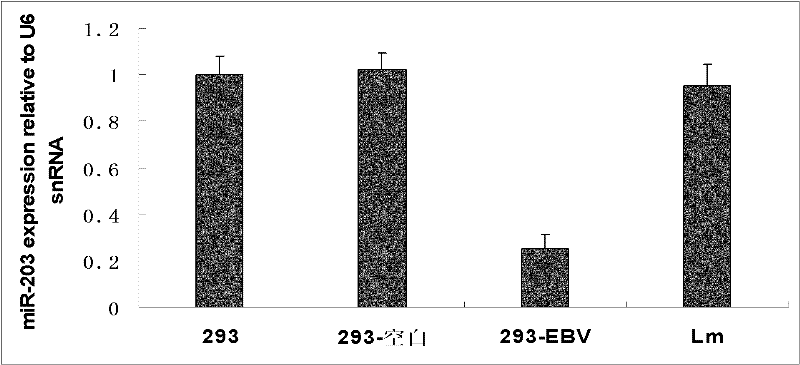
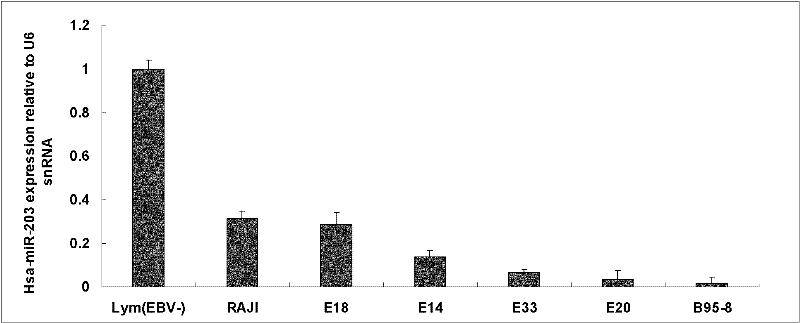
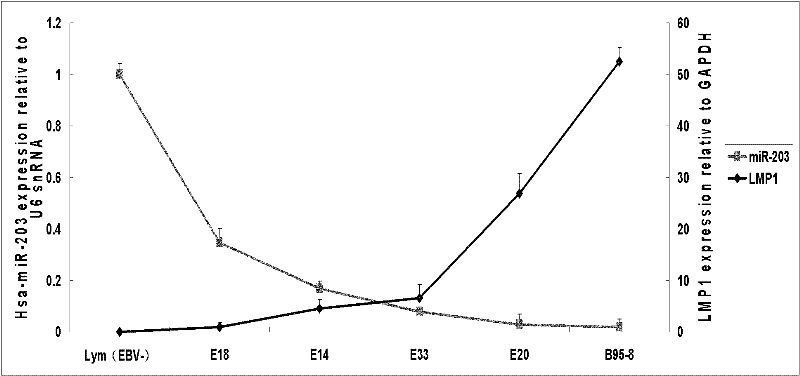
The invention discloses a molecular target hsa-miR203 for diagnosing and treating nasopharyngeal cancer related with Epstein-Barr virus (EBV) infection and application thereof. The microRNA can accurately reflect the conditions of retention and loss of EBV viruses in cells and tissues, and further can be used for diagnosing the nasopharyngeal cancer related with the EBV infection; and meanwhile, target genes E2F3, CCNG1 and the like controlled by the hsa-miR203 obviously inhibit the proliferation and conversion capacities of the cells, so the hsa-miR203 can be used for treating the nasopharyngeal cancer related with the EBV infection. Therefore, the hsa-miR203 has important scientific research theory and clinical application value, and provides new clue and proof for diagnosis, treatment or prognosis of EBV related tumors.
Owner:CENT SOUTH UNIV
Cytotoxic t-cell epitope peptide and use thereof
InactiveCN107709351AMicrobiological testing/measurementImmunoglobulins against animals/humansImmunotherapeutic agentEBV Infections
The present invention provides a cytotoxic T-cell (cytotoxic T lymphocyte, abbreviated hereinafter as CTL) epitope peptide specific to the Epstein-Barr virus (described hereinafter as EBV), a vaccinefor treating or preventing EBV infection or EBV-positive cancer using this peptide, a passive immunotherapeutic agent for EBV, and a method for assaying CTL specific to EBV. The present invention alsoprovides an HLA-A*24:02-restricted epitope peptide comprising an IYTEVRELV sequence (SEQ ID NO: 43) from a cytoskeleton-associated protein (cytoskeleton-associated protein 4: CKAP4 hereinafter, alsoknown as: CLIMP-63, ERGIC-63, P63). The peptide-specific cytotoxic T-cells (CTL hereinafter) can attack malignant tumor cells that express a high level of CKAP4.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
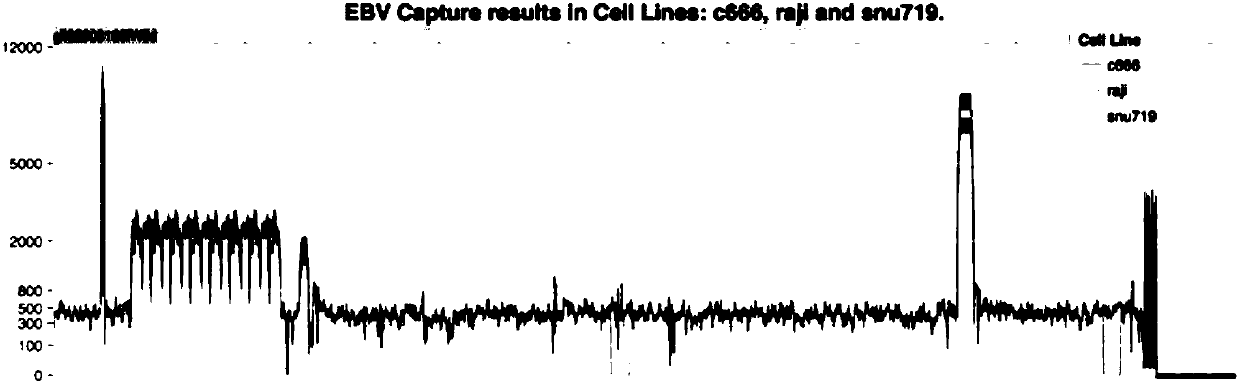
EBV capture probe and method for acquiring EBV genome sequence information in sample
InactiveCN107858451AAchieve enrichmentAvoid it happening againMicrobiological testing/measurementDNA/RNA fragmentationDiseaseEbv infection
The invention provides an EBV capture probe and a method for acquiring EBV genome sequence information in a sample. The capture probe designed by the invention has the following advantages: the capture efficiency on various EBV infection-associated diseases such as lymphoma, nasopharynx cancer and gastric cancer as well as EBV-containing cell lines (C666, Raji and snu179) is relatively high, the coverage degree is close to 100 percent and the sequencing data coverage is uniform, so that the chip can capture the information of the EVB genome well and has important significance in deep researchon the virus.
Owner:SUN YAT SEN UNIV CANCER CENT
Ex vivo method for producing a preparation containing cd4+ t cells specific for ebv structural antigens
ActiveUS20110059133A1Efficient expansionEfficiently presentedViral antigen ingredientsBlood/immune system cellsAntigenEbv infection
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT +1
Ex vivo method for producing a preparation containing CD4+ T cells specific for EBV structural antigens
ActiveUS9028840B2Efficient expansionEfficiently presentedViral antigen ingredientsBlood/immune system cellsAntigenEbv infection
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT +1
Method for establishing NK and/or T cell line
InactiveCN108251368AMicrobiological testing/measurementAntiviralsHigh concentrationLymphocyte proliferation
The invention discloses a method for establishing a cell line, and discloses a method for establishing a NK and / or T cell line. The method is specifically as follows: collecting peripheral blood mononuclear cells of patients with chronic active EBV infection or other EBV-infection-related lymphocyte proliferation, lymphoma or leukemia, and culturing the peripheral blood mononuclear cells in a medium containing human serum in the presence of cytokines. According to the method, NK and or T cells which can proliferate continuously can be established from a small amount of the peripheral blood mononuclear cells, and the obtained cell line can be continuously cultured for more than 3 months. The method requires only the small amount of the peripheral blood mononuclear cells, does not require use of expensive equipment and high concentrations of the cytokines, and can significantly improve the efficiency of establishing of cell lines.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Epstein Barr virus antibodies, vaccines, and uses of the same
ActiveUS11116835B2Reduce riskReduce infectionViral antigen ingredientsImmunoglobulins against virusesEpstein-Barr Virus AntibodyMononucleosis
Anti-Epstein Barr Virus (EBV) antibodies and vaccines are described herein. The antibodies and vaccines can be used to treat and / or reduce the risk of EBV infection and to treat and / or reduce the risk of complications associated with EBV infection, such as infectious mononucleosis, lymphoproliferative disorders, carcinomas, and smooth muscle tumors.
Owner:FRED HUTCHINSON CANCER CENT
Peptides derived from capsid antigens of the Epstein-Barr virus and the use thereof
ActiveUS20060057563A1Safe differentiationSugar derivativesMicrobiological testing/measurementAntigenEpstein bar virus
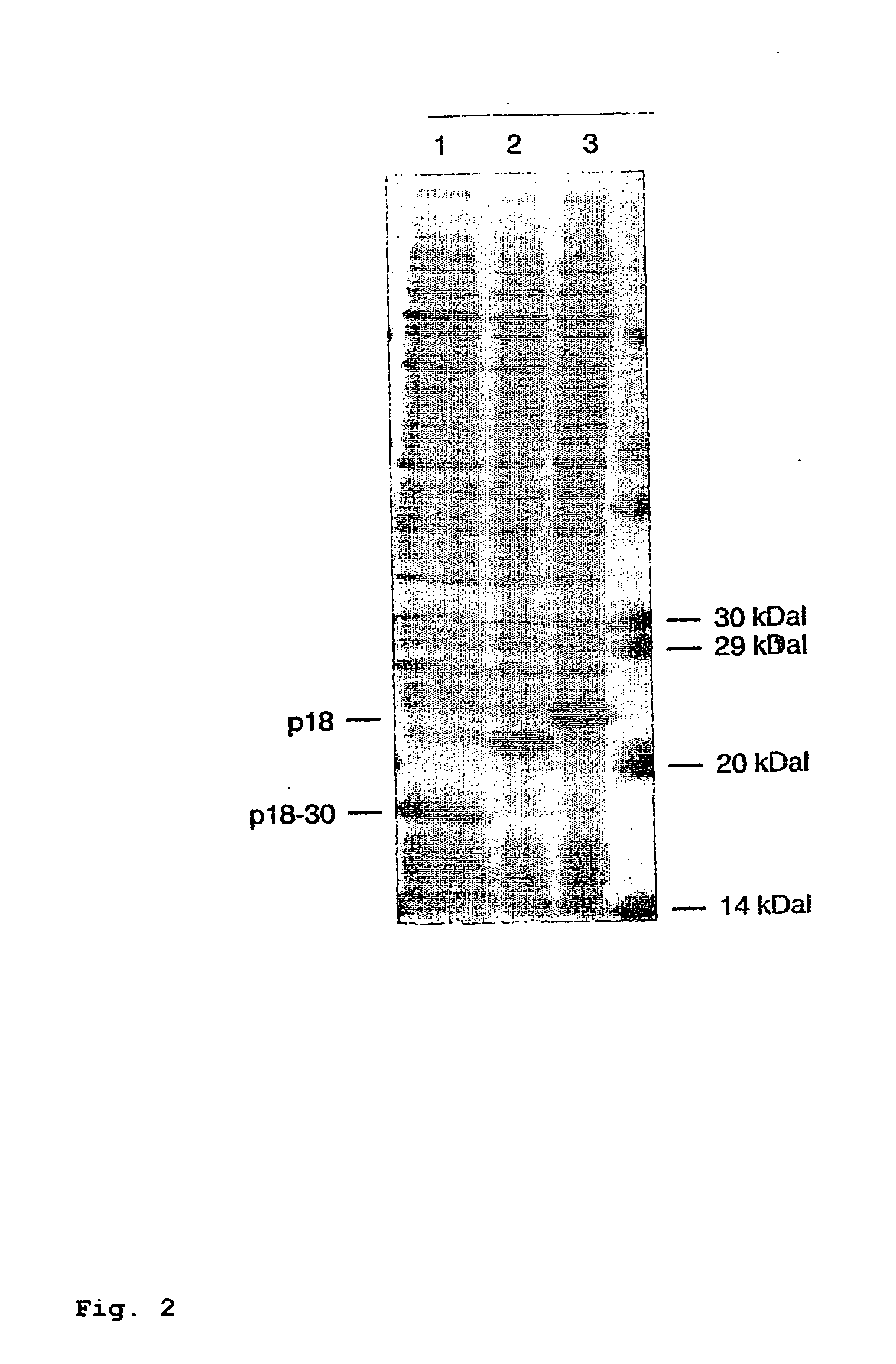
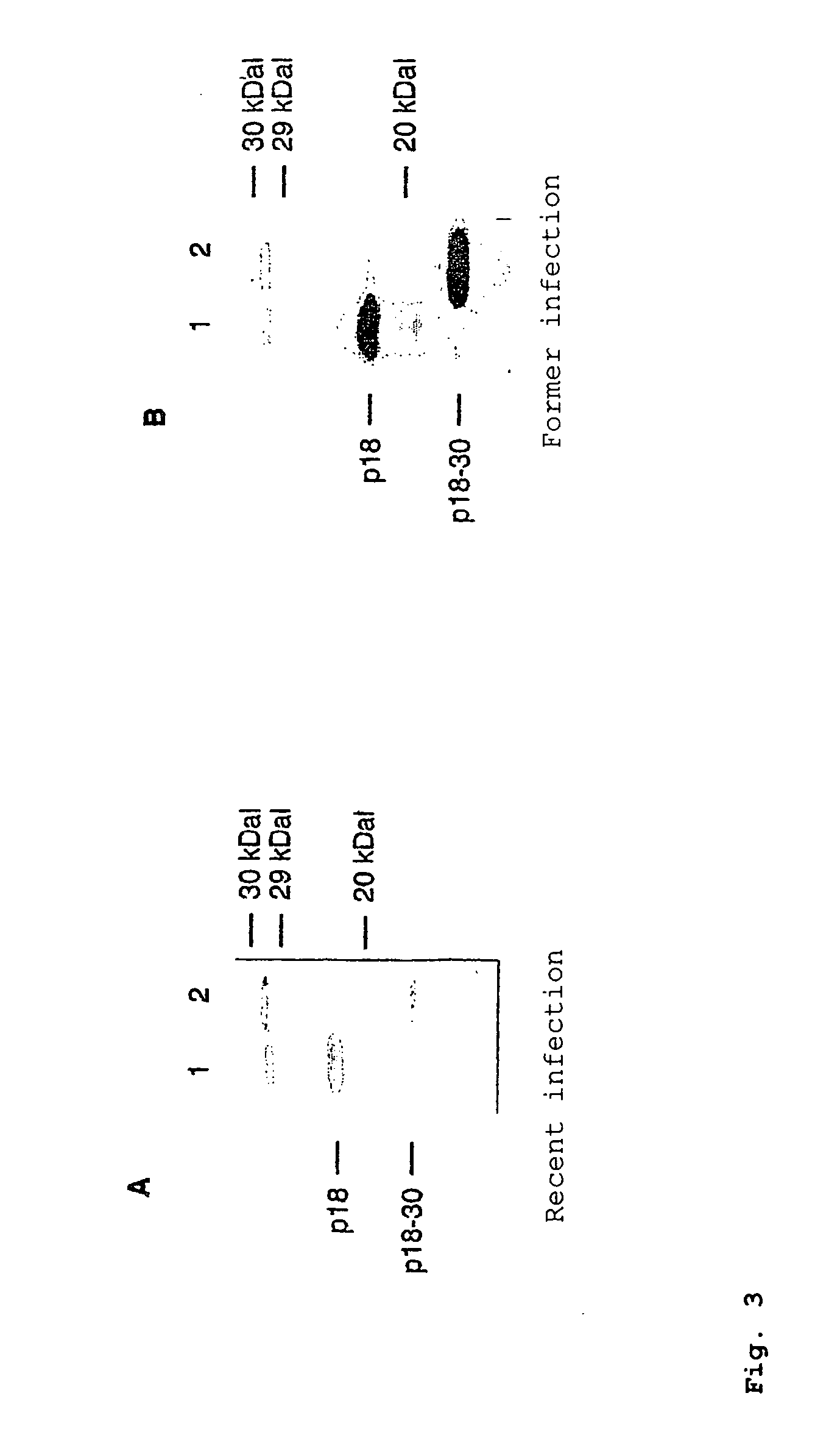
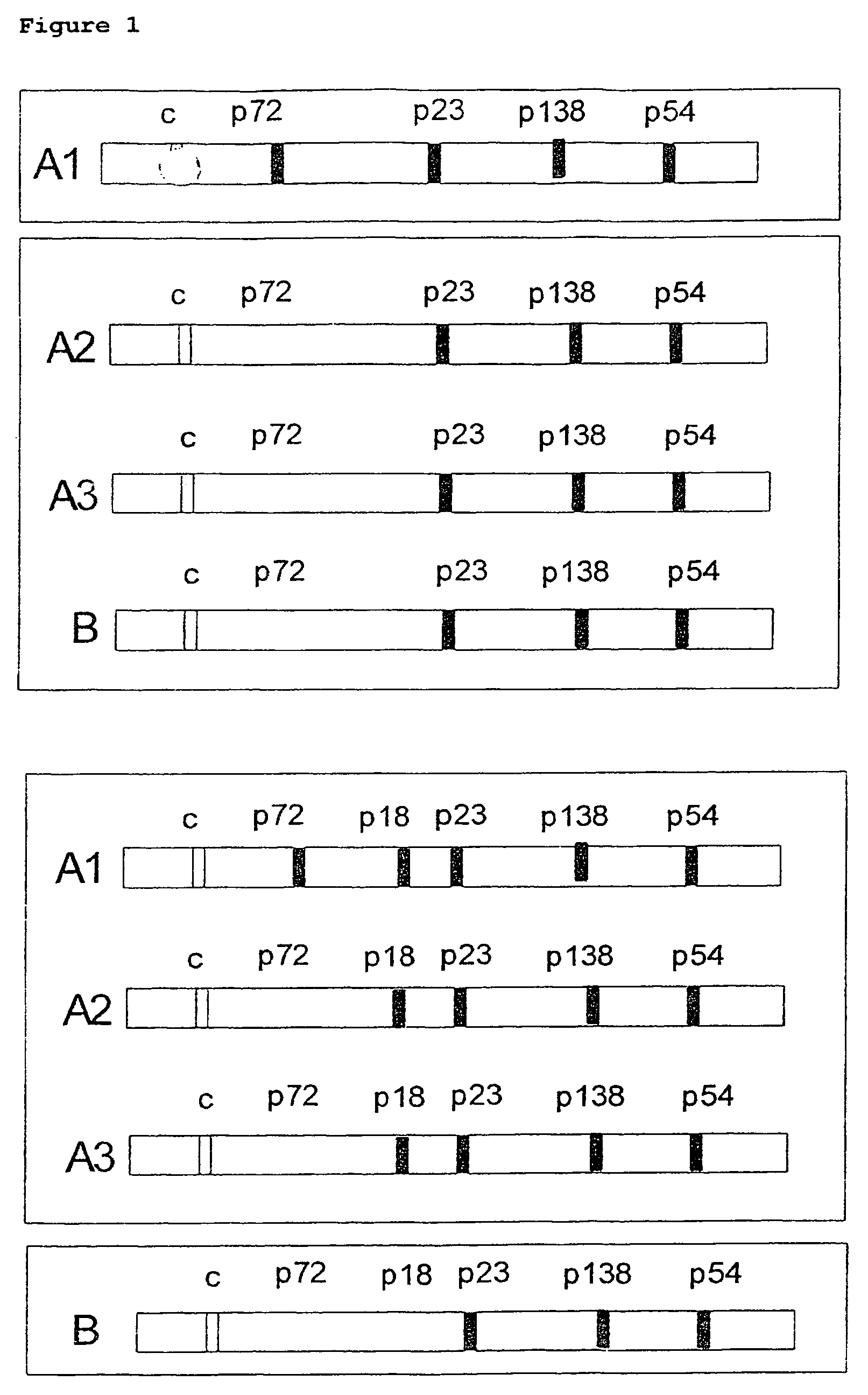
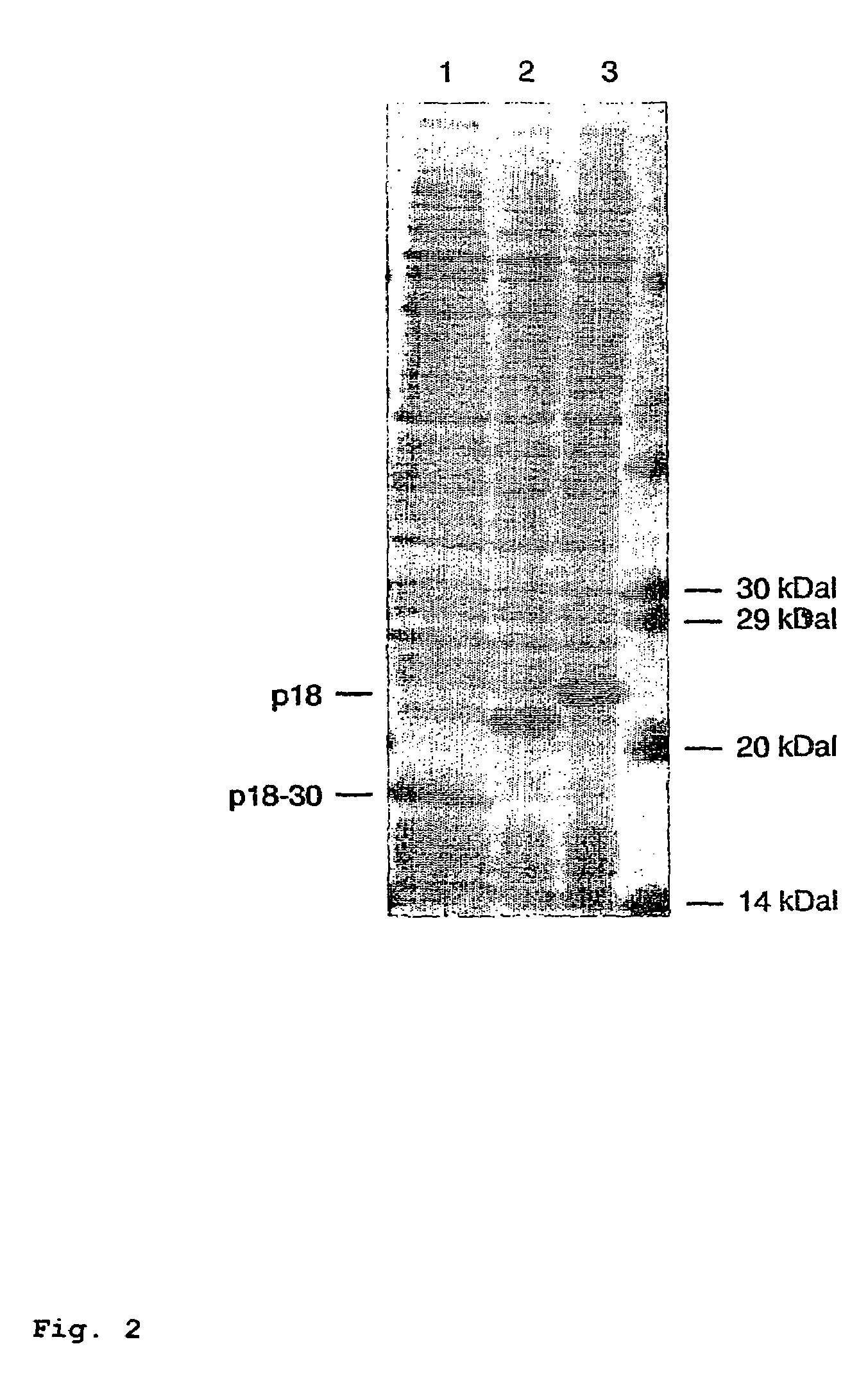
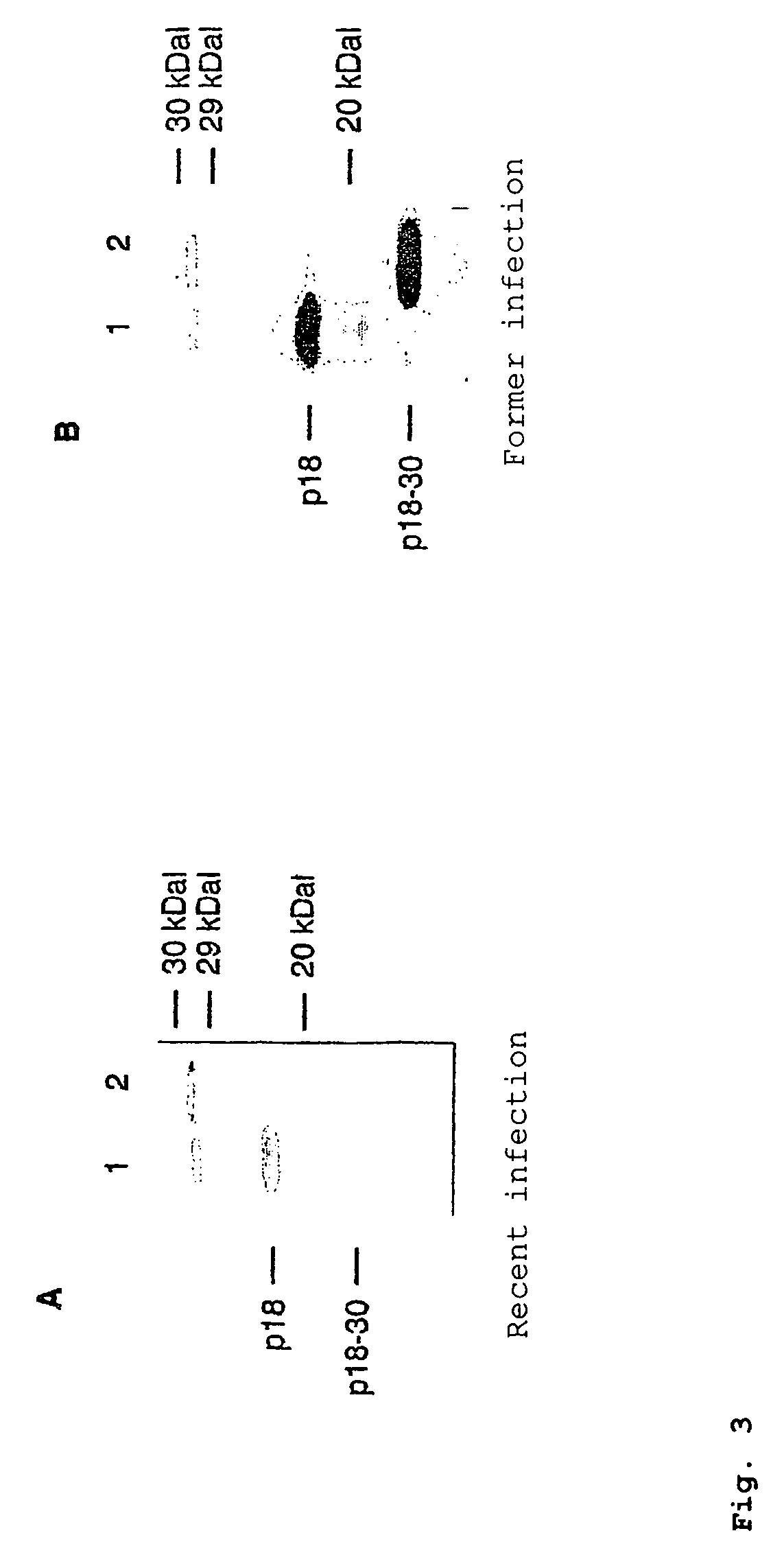
In the area of virus diagnosis, in particular Epstein-Barr virus (EBV) diagnosis, methods for the detection of EBV and agents suitable for this purpose are provided, including peptides which are derived from p18-VCA and permit discrimination between acute and past EBV infection.
Owner:MIKROGEN MOLEKULARBIOLOGISCHE ENTWICKLUNGS GMBH
NK cell line of human
ActiveCN108220236AGood antitumor activityMicrobiological testing/measurementMammal material medical ingredientsDiseaseLymphocyte proliferation
The invention relates to the cell line, and specifically relates to a NK cell line of the human. The conservation number is CGMCC No. 14891. The NK cell line of the human provided by the invention canbe passaged for a long time and greatly amplified, and has stronger anti-tumor activity, and provides a new experimental model for deeply development chronic activity EBV infectious disease mechanismresearch, screening chronic activity EBV infection, treatment medicine of EBV related lymphocytosis and the like, natural killer cell anti-tumor action mechanism and clinic adoptive immunity treatment for a researcher.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Epstein barr virus antibodies, vaccines, and uses of the same
ActiveUS20200164059A1Reduce EBV infectionReduce riskViral antigen ingredientsImmunoglobulins against virusesEpstein-Barr Virus AntibodyMononucleosis
Anti-Epstein Barr Virus (EBV) antibodies and vaccines are described herein. The antibodies and vaccines can be used to treat and / or reduce the risk of EBV infection and to treat and / or reduce the risk of complications associated with EBV infection, such as infectious mononucleosis, lymphoproliferative disorders, carcinomas, and smooth muscle tumors.
Owner:FRED HUTCHINSON CANCER CENT
Assay method for EBV (Epstein-Barr virus)
PendingCN107723383AGuaranteed catchHigh sensitivityMicrobiological testing/measurementMicroorganism based processesDiseaseAssay
The invention discloses a high-throughput sequencing assay method for Epstein-Barr virus (EBV) integration assay. The method utilizes the genome information of the Epstein-Barr virus (EBV) in combination with the second-generation high-throughput sequencing technology to more comprehensively assay the condition of EBV infection of a patient, overcoming the defects of conventional assay methods, such as low accuracy, high false positive and poor repetitiveness. The adoption of gene sequencing can help increasing accuracy, and compared with the classical Sanger sequencing method mostly adopted at present, the assay method has the advantages of higher assay throughput, higher accuracy, lower cost, richer information and the like. With the help of the second-generation high-throughput sequencing technology, the assay method can carry out high-precision assay on the human infection of the Epstein-Barr virus, so that specific treatment can be carried out at the infection stage to reduce theharm of the disease on the human body, and thereby the occurrence of tumor is decreased.
Owner:JIAXING YUNYING MEDICAL INSPECTION CO LTD
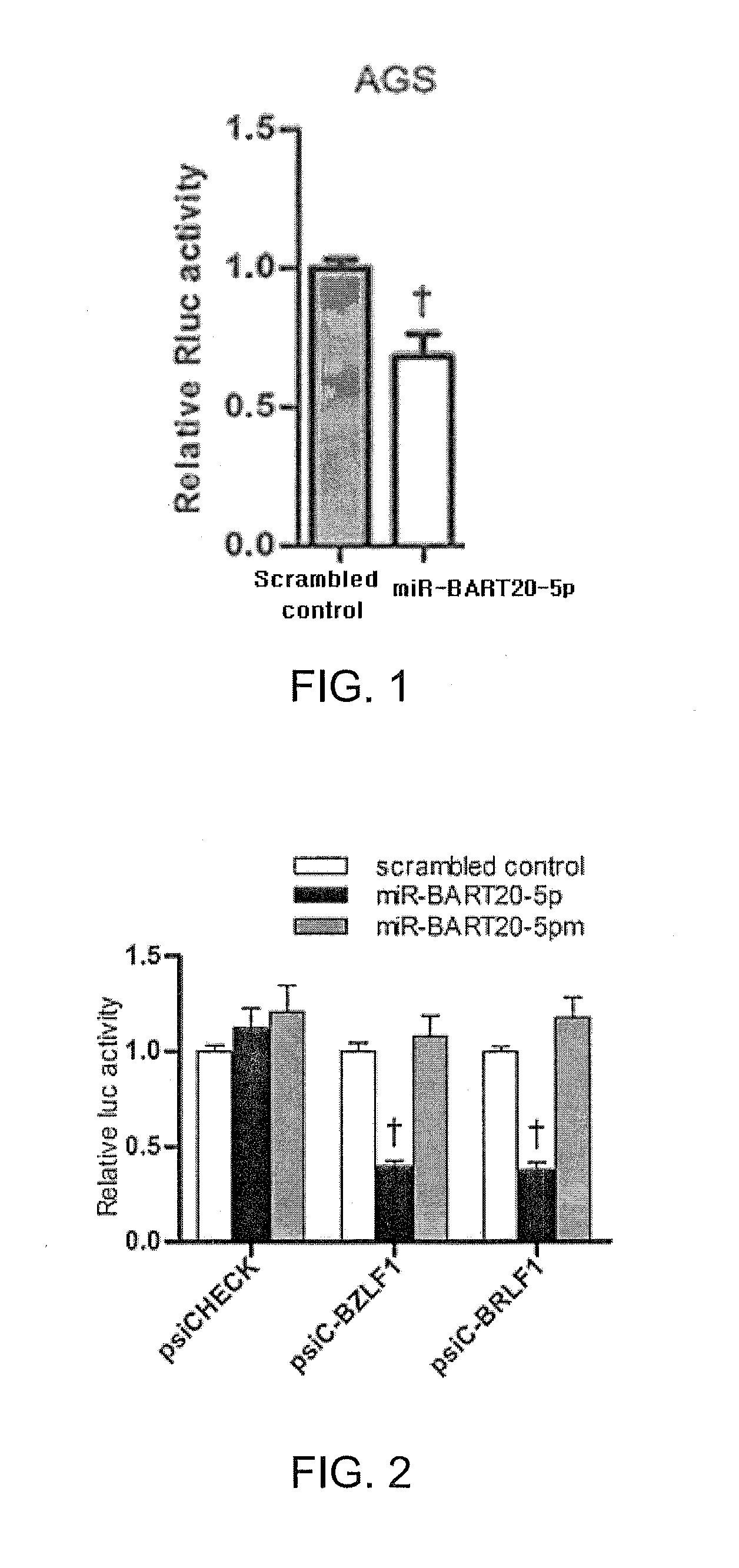
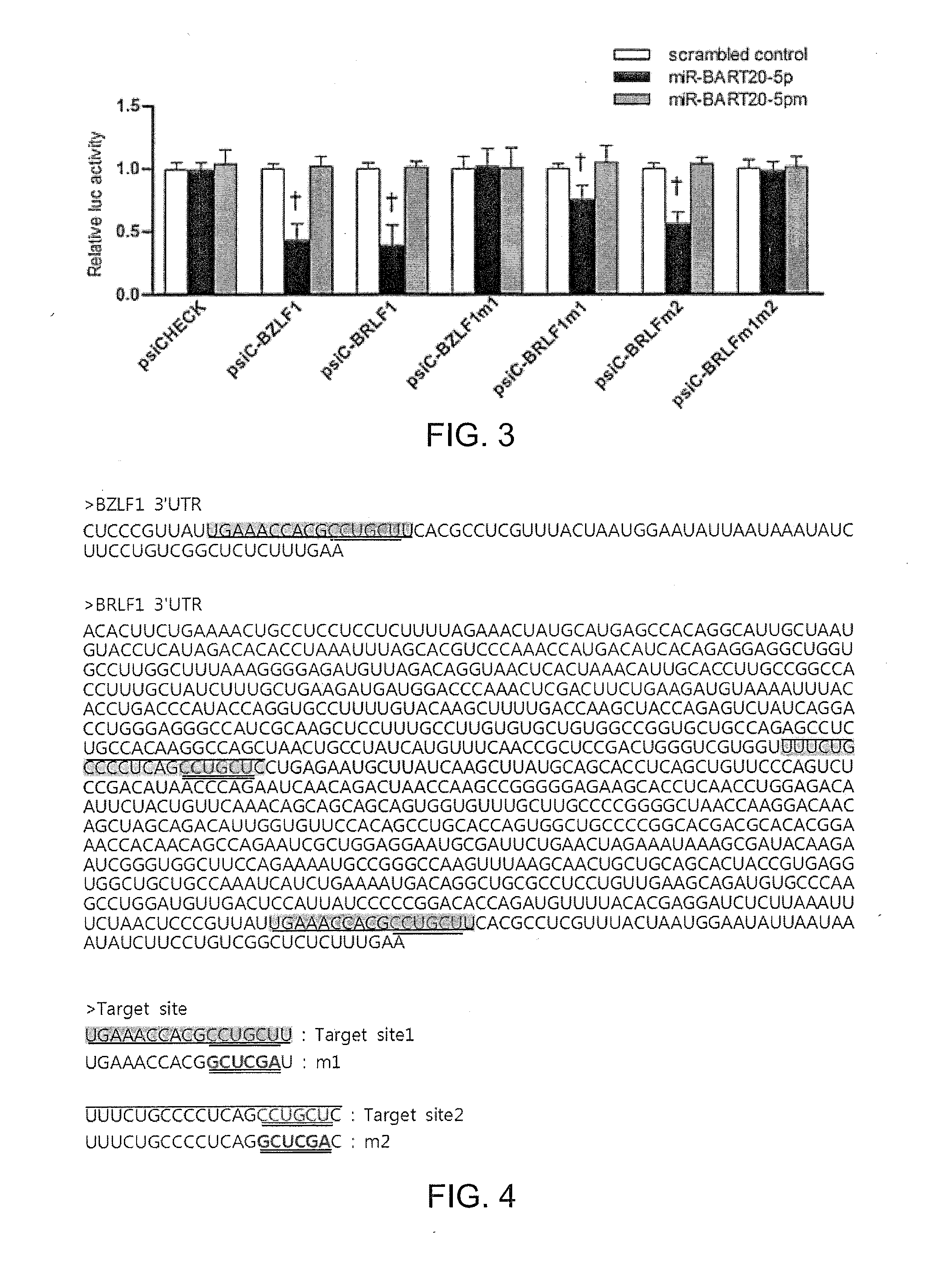
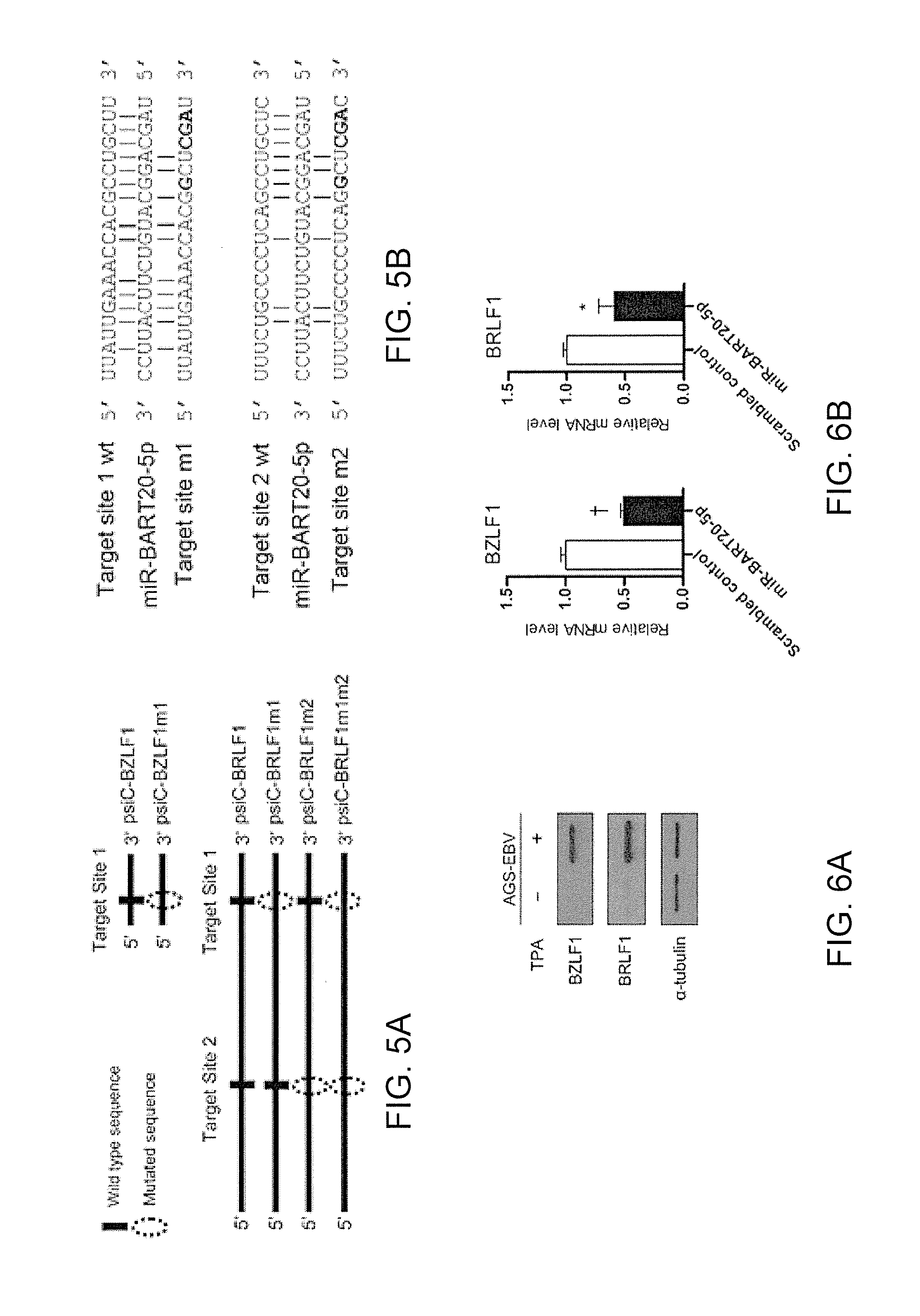
Composition for treating epstein-barr virus infection, comprising epstein-barr virus micro RNA inhibitor
InactiveUS20150329865A1Good antiviral effectEffective preventionMicrobiological testing/measurementInactivation/attenuationDiseaseMicroRNA
Provided is a composition comprising an Epstein-Barr virus microRNA inhibitor for treating Epstein-Barr virus infection, and a method using Epstein-Barr virus microRNA for screening a therapeutic agent for treating Epstein-Barr virus infection. The provided composition enables one to induce the lytic cycle of EBV such that EBV-infected cells are destroyed by a host immune system. Therefore, the composition can be effectively used for the prevention or treatment of diseases, including various cancers, caused by EBV infection. Moreover, the provided method enables one to screen a therapeutic agent having excellent antiviral effect for treating Epstein-Barr virus infection by inducing Epstein-Barr virus lytic cycle.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
NK/T cell line of human
ActiveCN108220237AGood antitumor activityMicrobiological testing/measurementMammal material medical ingredientsDiseaseAbnormal tissue growth
The invention relates to a cell line, and specifically relates to a NK / T cell line of the human. The conservation number is CGMCC No. 14892. The NK / T cell line of the human provided by the invention can be passaged for a long time and greatly amplified, and has stronger anti-tumor activity, and provides a new experimental model for deeply development chronic activity EBV infectious disease mechanism research, screening chronic activity EBV infection, diagnosis molecular marker of EBV related lymphocytosis and the like, treating prognosis maker and treatment medicine, CD8+T cell, natural killercell anti-tumor action mechanism and clinic adoptive immunity treatment for a researcher.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
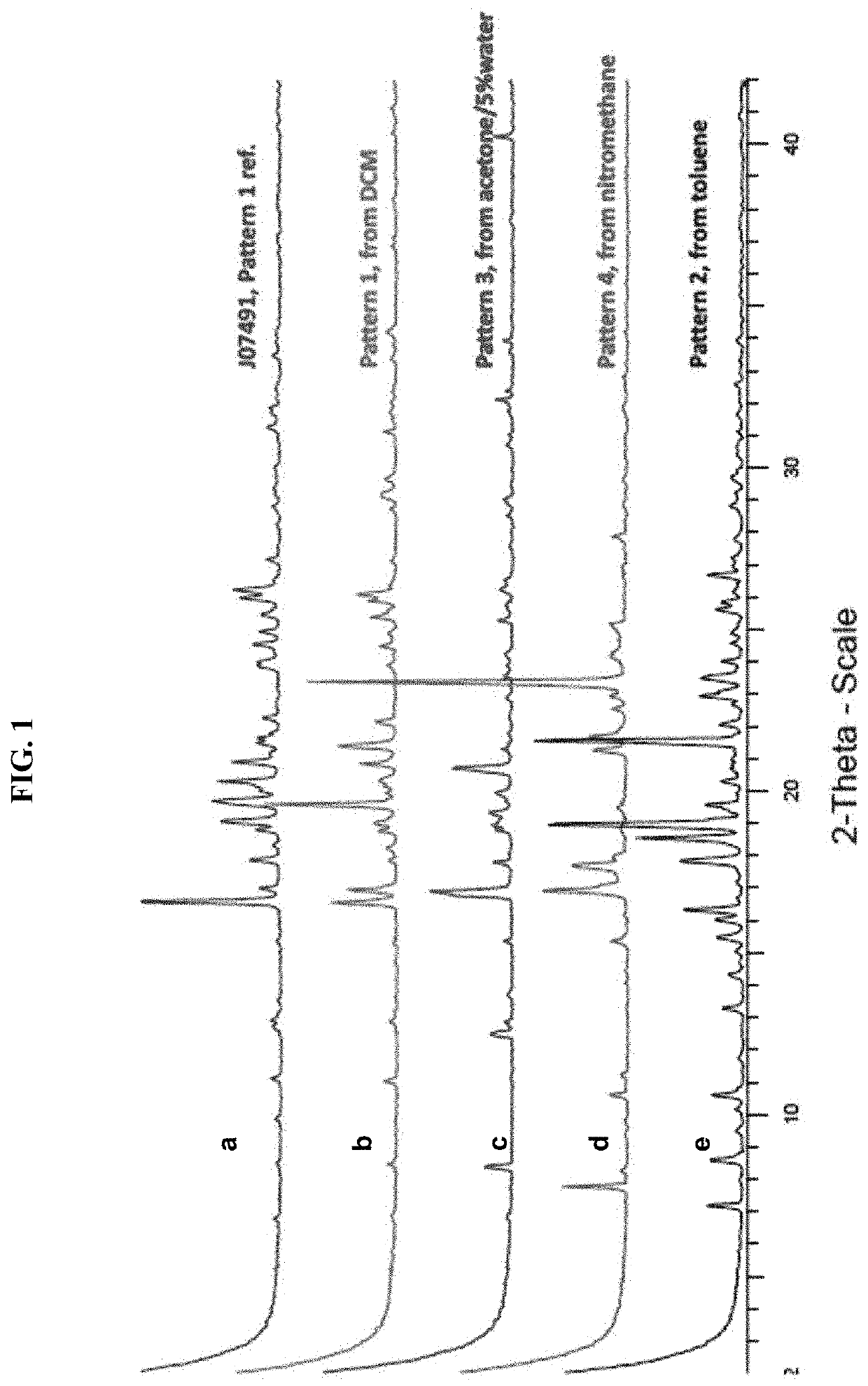
EBNA1 Inhibitor Crystalline Forms, and Methods of Preparing and Using Same
ActiveUS20190352285A1Avoid infectionOrganic active ingredientsNervous disorderMedicineEBV Infections
The invention relates, in certain aspects, to developable forms of certain compounds that are useful to treat and / or prevent EBV infection and related conditions in a subject. The invention further provides EBNA1 inhibitors, and / or pharmaceutical compositions comprising the same, that are useful for the treatment of diseases caused by latent Epstein-Barr Virus (EBV) infection and / or lytic EBV infection.
Owner:WISTAR INSTITUTE
EBNA1 inhibitors and methods using same
The present invention provides EBNA1 inhibitors, and / or pharmaceutical compositions comprising the same, that are useful for the treatment of diseases caused by EBNA1 activity, such as, but not limited to, cancer, infectious mononucleosis, chronic fatigue syndrome, multiple sclerosis, systemic lupus erythematosus and / or rheumatoid arthritis. The present invention further provides EBNA1 inhibitors, and / or pharmaceutical compositions comprising the same, that are useful for the treatment of diseases caused by latent Epstein-Barr Virus (EBV) infection and / or lytic EBV infection.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY
EBER probe for detecting EBV infected tissue and detection kit
PendingCN113151588AGood hybrid specificityThe test result is accurateMicrobiological testing/measurementMicroorganism based processesEBV InfectionsNucleotide
The invention provides an EBER probe for detecting EBV infected tissues and a detection kit, and belongs to the technical field of virus detection. The EBER probe is a fluorescein-labeled EBER probe, and the nucleotide sequence of the EBER probe is shown in SEQ ID NO: 1. The probe hybridization solution is an aqueous solution containing 10-20 pmol / [mu]l of an EBER probe, 50% of formamide, 10% of dextran sulfate, 1.0% of Triton X-100 and 50 mmol / L of Tris-HCl. The invention also provides the detection kit which comprises the probe hybridization solution, a DAPI counterstaining agent and a quality control sheet group, can realize the detection of the EBER state in a tissue or cell sample, and assists in clinically judging whether EBV infection exists or not. Through verification, the kit is high in detection sensitivity and good in specificity, the detection method is simple and convenient, the defects of an existing product or method are overcome, and the kit has wide application prospects.
Owner:GUANGZHOU LBP MEDICINE SCI & TECH
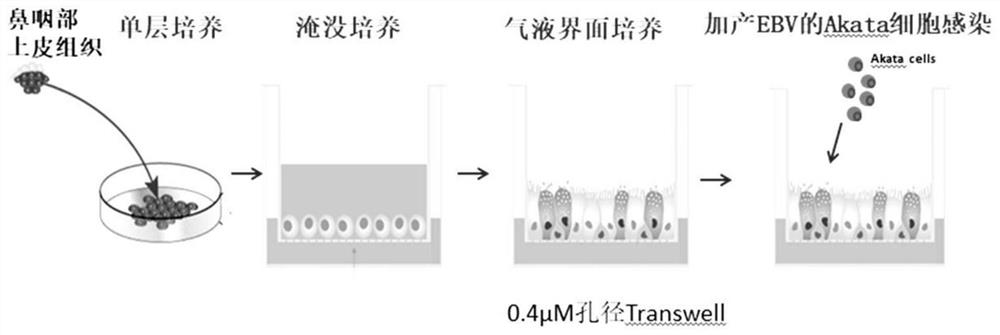
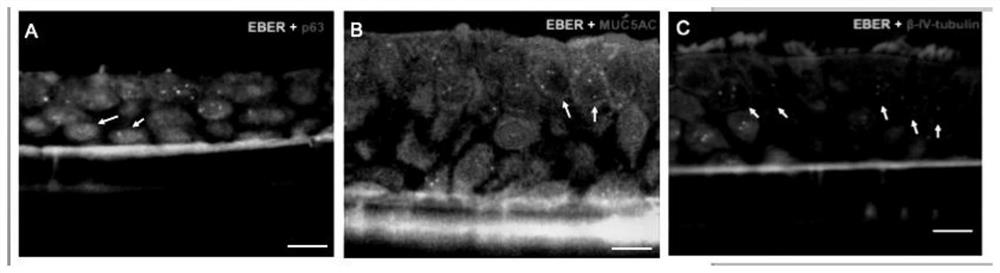
Method for establishing EBV virus infected artificial respiratory tract epithelium model
The invention discloses a method for establishing an EBV (Epstein-Barr Virus) infected artificial respiratory tract epithelium model. The method comprises the following steps: (1) digesting nasopharyngeal mucosa tissues by using Dispase II, performing beating into single cells, and performing centrifuging to remove supernate, so as to obtain cell precipitate; (2) suspending and culturing the cellprecipitate by using an epithelial cell culture medium; (3) performing digesting into single cells, planting the single cells in a small chamber above the 24-hole support membrane, and performing culturing until the support membrane is overgrown; (4) completely removing the culture solution above the support membrane, and continuously culturing for 2-3 weeks; and (5) when large-area cilium swinging is observed, adding Akata cells to carry out cell contact mediated EBV infection, and when successful infection, obtaining the artificial respiratory tract epithelium model infected with the EBV. According to the establishing method, nasopharyngeal mucosa tissue is taken as a material part, nasopharyngeal epithelial cells can be differentiated into completely polarized pseudo-multilayer respiratory tract epithelial tissue through gas-liquid interface culture, and the nasopharyngeal epithelial tissue is completely simulated by basal layer cells, secretory cuppy cells and swinging cilium cells.
Owner:山东银丰生命科学研究院 +1
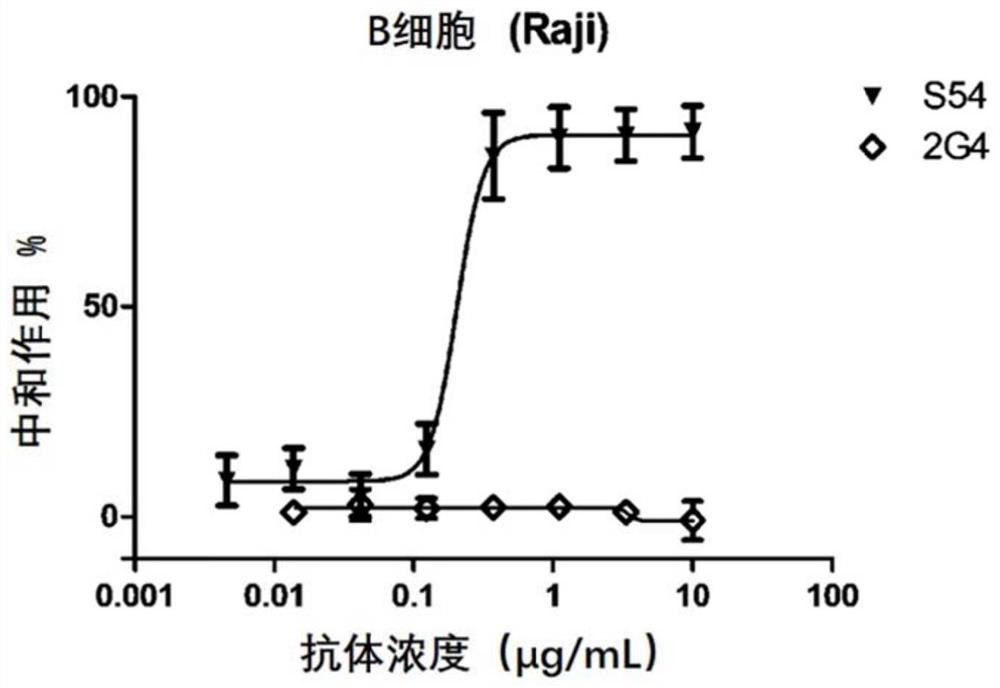
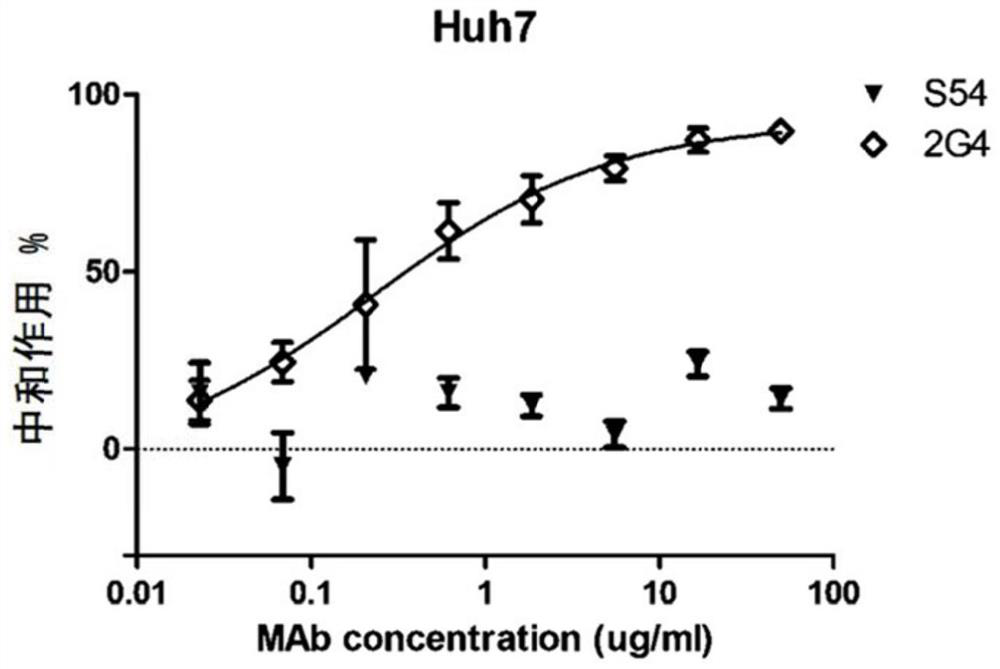
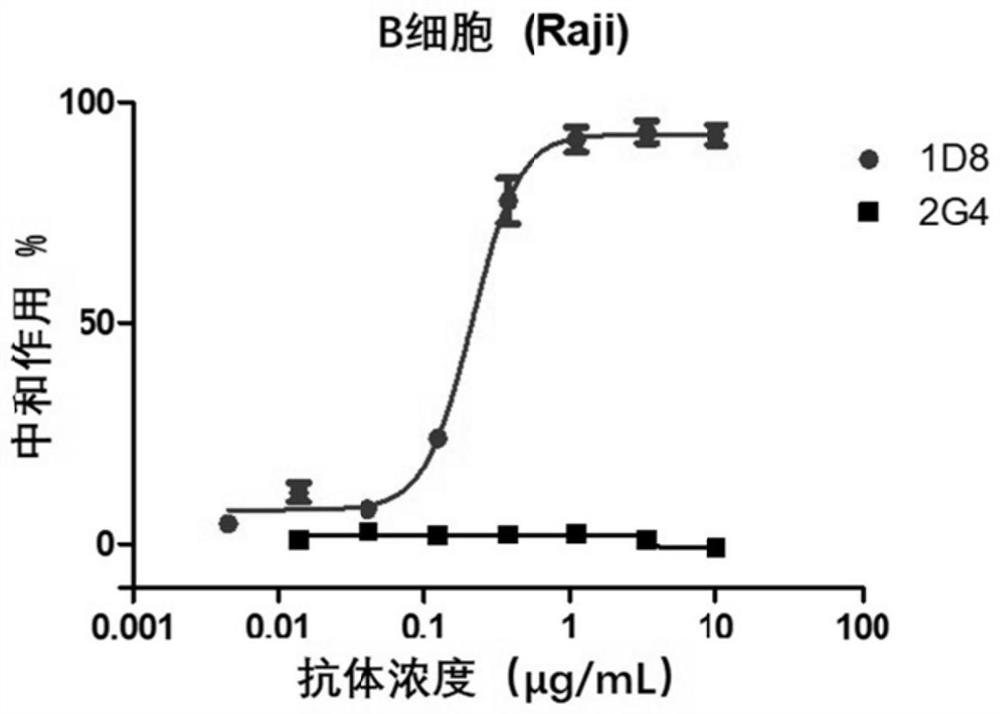
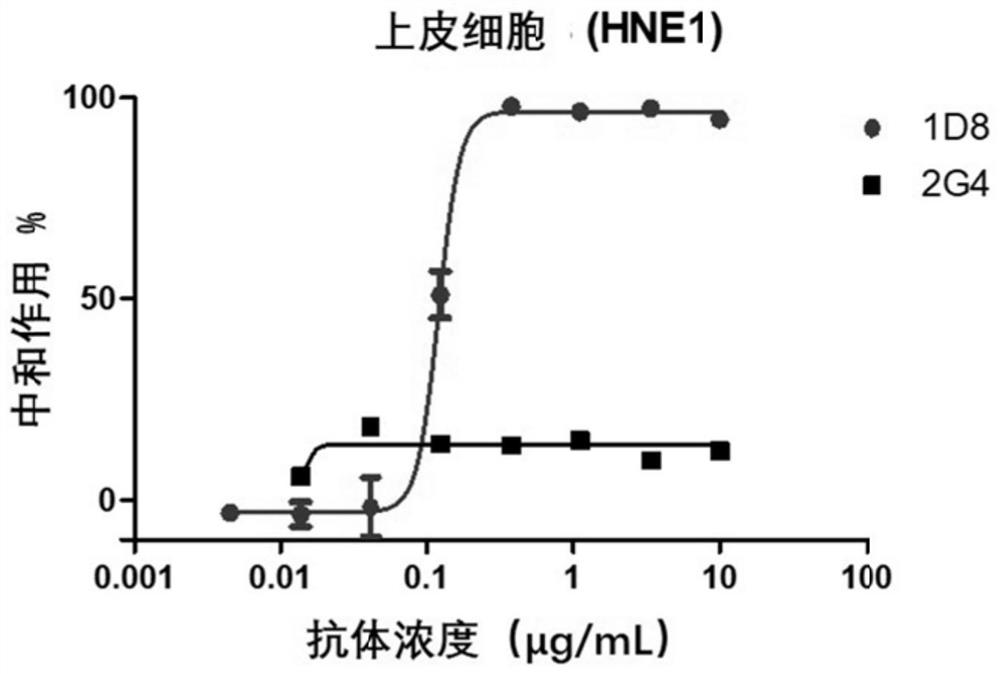
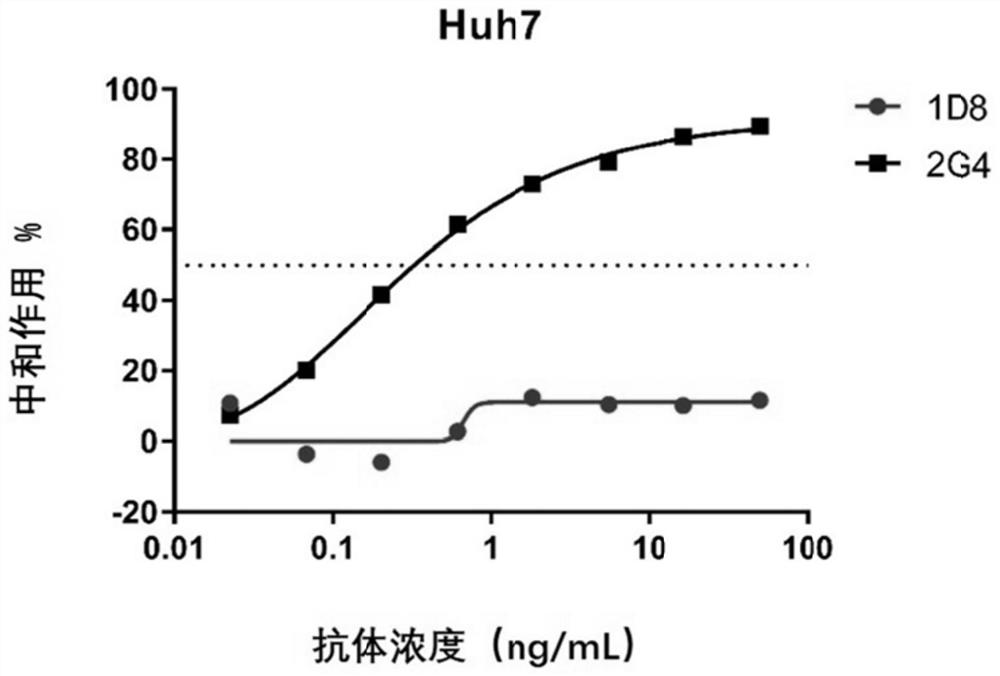
A kind of monoclonal antibody and application thereof for neutralizing Epstein-Barr virus
ActiveCN111574621BLittle side effectsImprove featuresImmunoglobulins against virusesAntiviralsHeavy chainEBV Infections
The present invention discloses an antibody against Epstein-Barr virus for the first time, which is composed of a light chain and a heavy chain. The heavy chain variable region of the heavy chain has three complementary regions CDR1, CDR2 and CDR3, and their amino acid sequences are respectively shown in SEQ ID NO .1-3, the light chain variable region of the light chain has 3 complementary regions CDR1', CDR2' and CDR3', the amino acid sequences of which are respectively as SEQ ID NO.4, GNN, SEQ ID NO.5 shown. The antibody can block EBV infection of B cells with an IC50 of 203ng / ml, but has no neutralizing effect on Ebola virus infection, indicating that the antibody has a high ability to specifically neutralize EBV infection of B cells.
Owner:SUN YAT SEN UNIV CANCER CENT +1
Traditional Chinese medicine compound extract for preventing and treating EBV (Ebola Virus) infection and preparation method thereof
InactiveCN107551227AGood curative effectSmall side effectsPharmaceutical delivery mechanismAntiviralsSide effectEbola virus
The invention discloses a traditional Chinese medicine compound extract prepared by processing astragalus, comfrey, Qingdai, scutellaria baicalensis, paeonol, angelica, zedoary and peach kernel. The traditional Chinese medicine compound extract can be used to prevent and treat EBV virus infection. The invention also discloses Application of the above-mentioned traditional Chinese medicine compound extract in the preparation of pharmaceutical preparations and health care products for preventing and treating EBV virus infection. The traditional Chinese medicine compound extract of the invention has good curative effect, less toxic and side effects, and low price, and is suitable for extensive clinical promotion.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
A kind of monoclonal antibody and application thereof for neutralizing Epstein-Barr virus
ActiveCN111690056BLittle side effectsImprove featuresImmunoglobulins against virusesAntiviralsEBV InfectionsEb virus
Owner:TSINGHUA UNIV +1
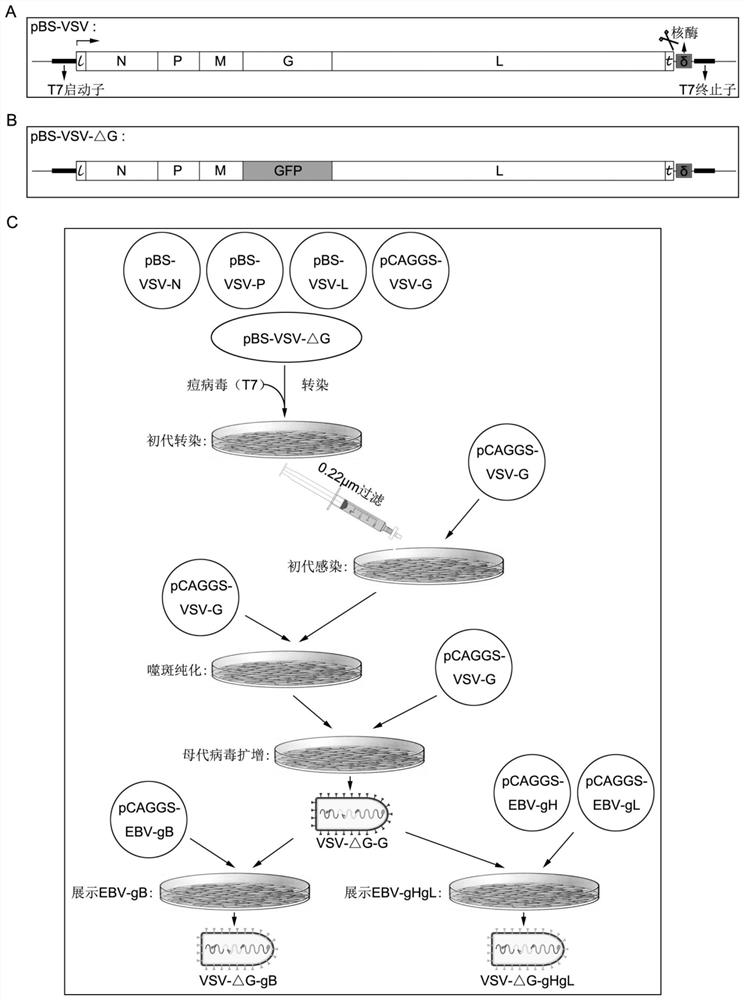
EBV vaccine based on vesicular stomatitis virus as well as preparation method and application of EBV vaccine
PendingCN114149979AReduce morbidityAvoid infectionSsRNA viruses negative-senseViral antigen ingredientsDiseaseEBV Infections
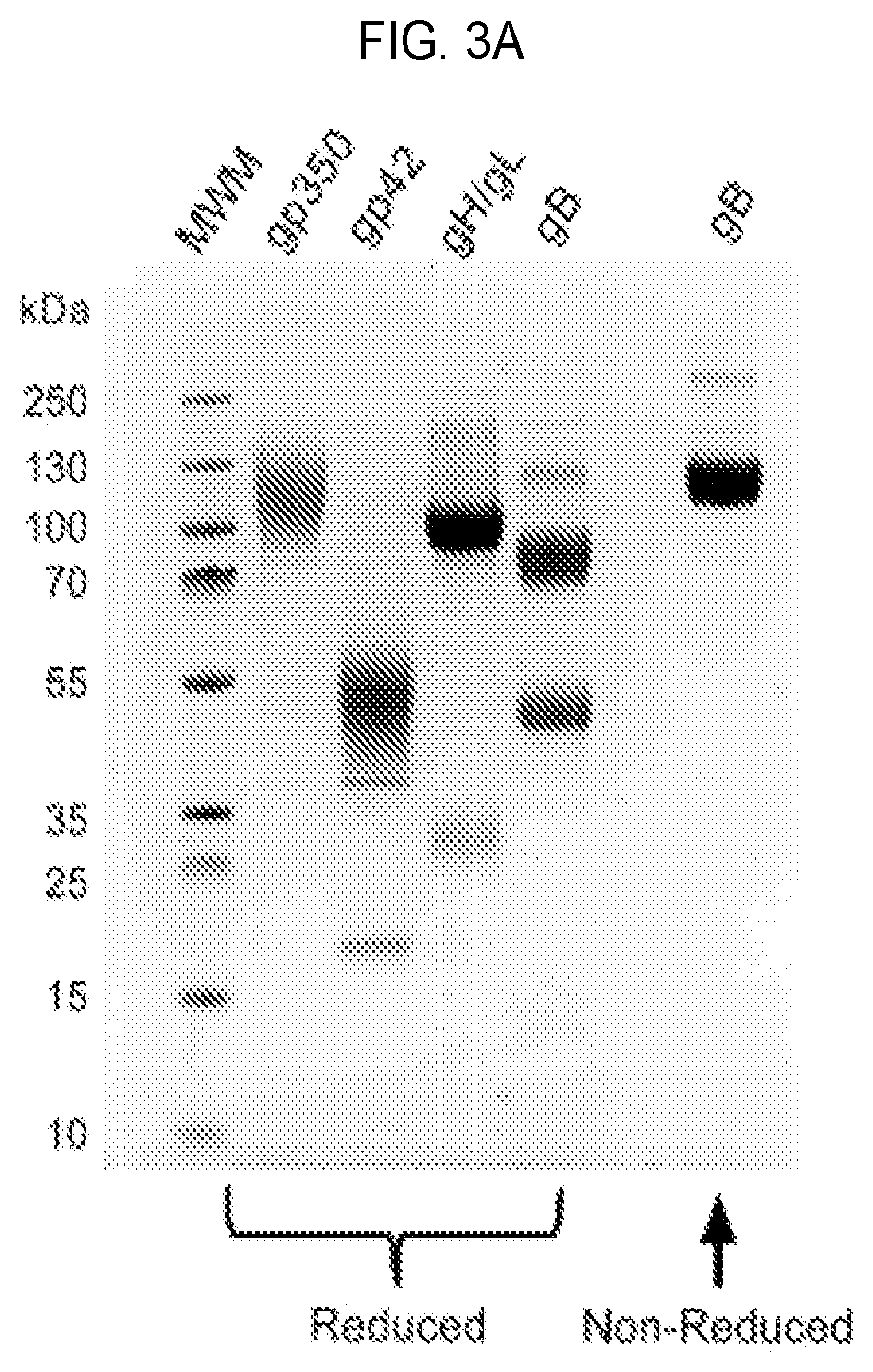
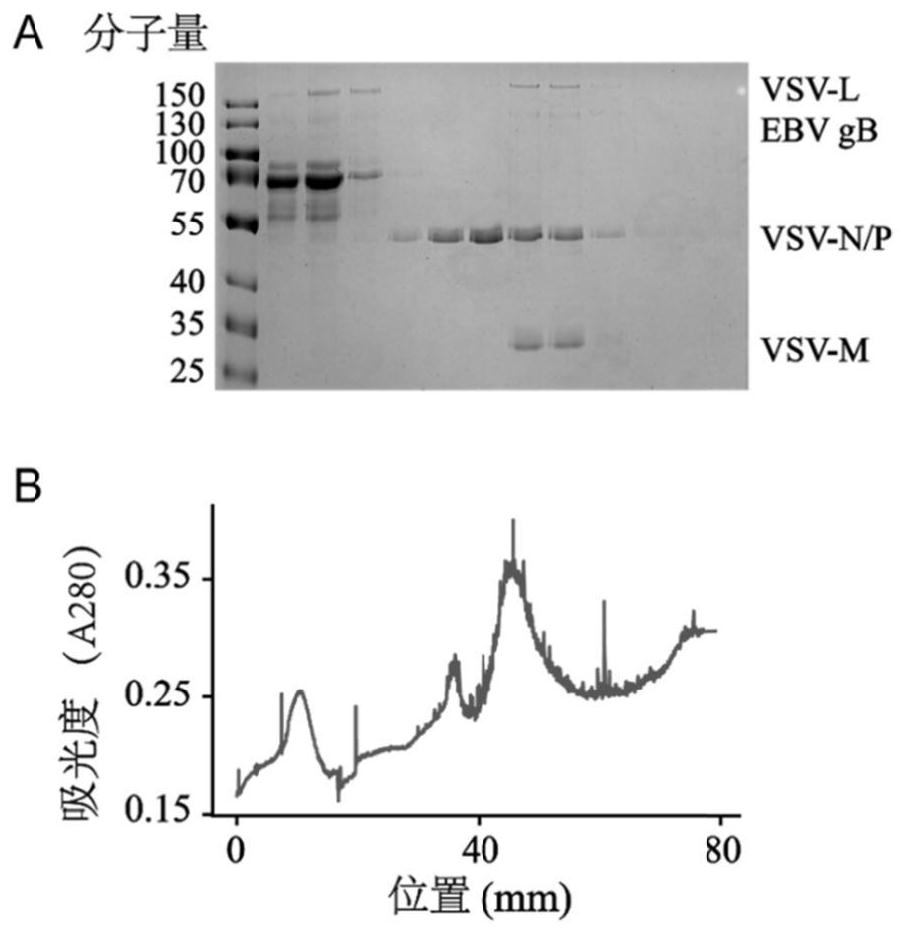
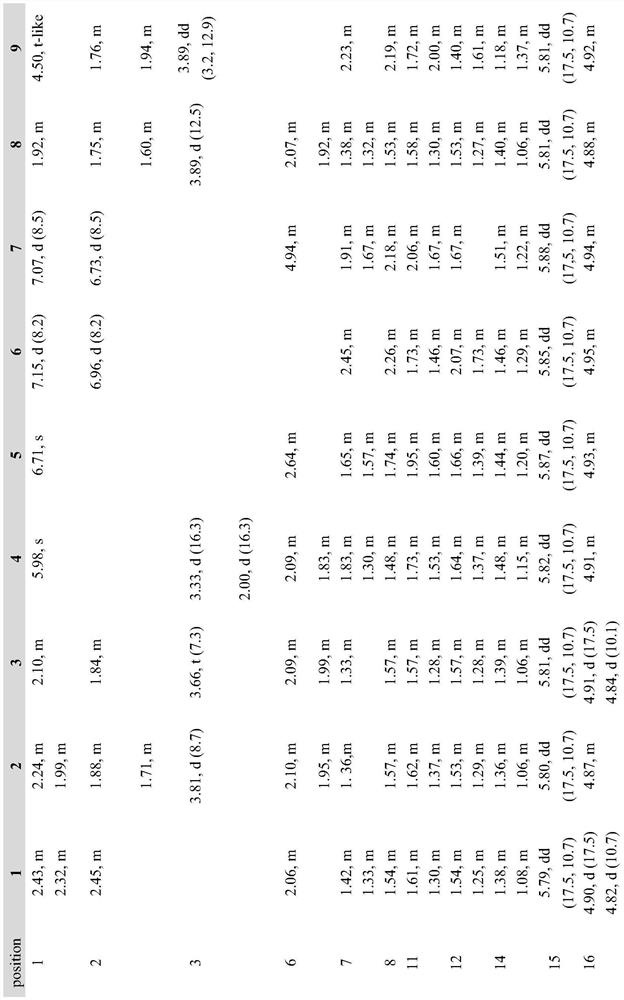
The invention discloses an EBV (Epstein-Barr Virus) vaccine based on a vesicular stomatitis virus as well as a preparation method and application of the EBV vaccine. EBV key glycoproteins gB and gHgL are displayed on the surface of VSV for the first time, the modified VSV is used for animal immunization, a new EBV vaccine variety is provided, an organism is expected to be induced to generate strong enough immune response to prevent EBV from infecting host cells, and therefore the morbidity of EBV-related neoplastic and non-neoplastic diseases is reduced. Detection finds that the modified VSV can induce obvious specific antibodies aiming at EBV surface glycoproteins gB and gHgL; in addition, it is proved that the generated specific antibody can inhibit EBV from infecting epithelial cells and B lymphocytes; the invention also shows that the EBV vaccine based on the VSV can be used for preventing and controlling EBV infection of people and related neoplastic and non-neoplastic diseases and has a good immune effect.
Owner:SUN YAT SEN UNIV CANCER CENT
A kind of iron crabapple extract and its preparation method and its application in the preparation and prevention of ebv virus infection medicine
ActiveCN106866401BLow toxicityEnhanced inhibitory effectOrganic compound preparationOrganic chemistry methodsAlcoholEBV Infections
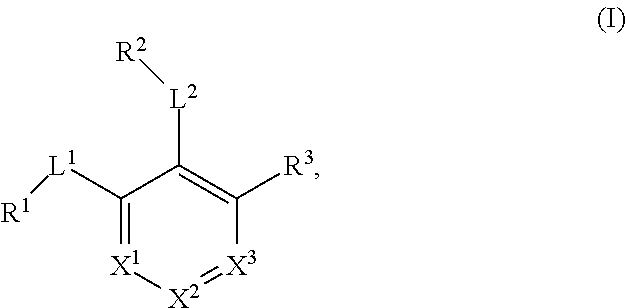
The invention discloses a euphorbia splendens extract. The structure formula of the euphorbia splendens extract is expressed as formula (I), wherein R1 and R2 are cyclized to form cyclohexene or phenyl; any one or more hydrogen groups on cyclohexene or phenyl are substituted by R4; R4 is selected from hydrogen, hydroxyl, carbonyl, C1-C5 alcohol group or C1-C5 alkyl; or R4 and carbon on cyclohexene or phenyl form carbonyl; R3 is hydrogen, hydroxyl or carbonyl. The euphorbia splendens extract provided by the invention has obvious inhibition effect on lytic replication split period of EBV, is relatively low in toxicity to host cells, has certain safety and can be used for preparing drugs for preventing and treating EBV infection. The formula (I) is shown in the description.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
A kind of synthetic ebv consensus sequence molecular dna vaccine and its application
The invention relates to three synthesized EBV (Epstein-Barr Virus) DNA (Deoxyribonucleic Acid) sequences which respectively encodes three consensus amino acid sequences EBNA1 (Epstein-Barr Nuclear Antigen 1), LMP1 (Latent Membrane Protein 1) and LMP2 (Latent Membrane Protein 2). The three sequences are respectively constructed on expression vector plasmids to express corresponding amino acid sequences. The invention further provides different methods for generating anti-corresponding EBV protein antigen immunoreaction by one or more expression plasmids.
Owner:杭州中晟纳博生物科技有限公司
A kind of monoclonal antibody and application thereof for neutralizing Epstein-Barr virus
ActiveCN111548411BLittle side effectsImmunoglobulins against virusesAntiviralsHeavy chainEBV Infections
Owner:SUN YAT SEN UNIV CANCER CENT +1
EBNA1 inhibitor crystalline forms, and methods of preparing and using same
The invention relates, in certain aspects, to developable forms of certain compounds that are useful to treat and / or prevent EBV infection and related conditions in a subject. The invention further provides EBNA1 inhibitors, and / or pharmaceutical compositions comprising the same, that are useful for the treatment of diseases caused by latent Epstein-Barr Virus (EBV) infection and / or lytic EBV infection.
Owner:WISTAR INSTITUTE
Peptides derived from capsid antigens of the Epstein-Barr virus and the use thereof
ActiveUS7476498B2Safe differentiationMicrobiological testing/measurementMaterial analysisAntigenEbv infection
In the area of virus diagnosis, in particular Epstein-Barr virus (EBV) diagnosis, methods for the detection of EBV and agents suitable for this purpose are provided, including peptides which are derived from p18-VCA and permit discrimination between acute and past EBV infection.
Owner:MIKROGEN MOLEKULARBIOLOGISCHE ENTWICKLUNGS GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com