EBV vaccine based on vesicular stomatitis virus as well as preparation method and application of EBV vaccine
A virus and vaccine technology, applied in the field of bioengineering, can solve the problems of reducing the incidence of infectious mononucleosis, unable to prevent EBV from infecting cells, and the clinical safety of granulated protein has not been effectively evaluated, so as to reduce the incidence of disease. rate, good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Packaging and amplification of embodiment 1VSV recombinant virus
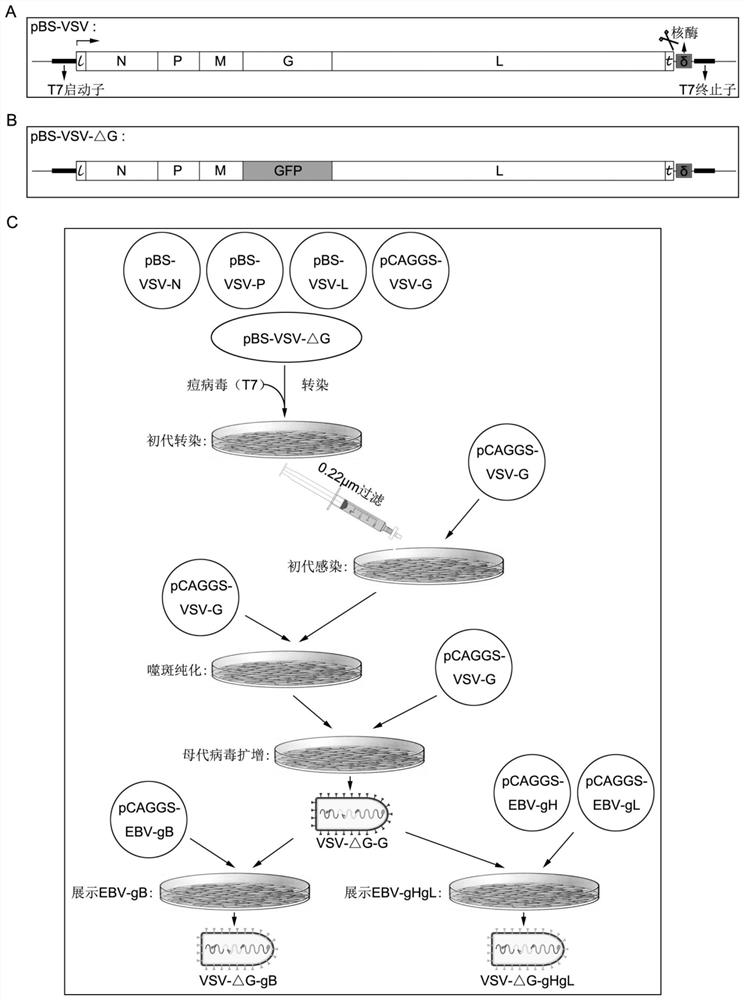
[0049] 1) Packaging and amplification of VSV recombinant virus see figure 1 . Among them such as figure 1 In A, the whole genome sequence of VSV was integrated into pBS plasmid vector (pBS-VSV) driven by T7 promoter. When the pBS-VSV plasmid is transfected into the cell, it starts to transcribe and synthesize the RNA chain under the action of T7 RNA polymerase, and is processed by the delta-ribozyme at the end of the RNA chain to obtain the complementary strand of the VSV genome, which is then used as The template is to synthesize the genomic RNA and mRNA of VSV by the RNA polymerase of VSV, and express each viral protein N, P, M, G, L, etc., and finally assemble the virus particles of VSV.
[0050] Such as figure 1 Shown in B, on the basis of pBS-VSV plasmid vector, remove the nucleotide sequence of the glycoprotein G of VSV, and replace it with the nucleotide sequence (pBS-VSV-△G) of GFP, the VSV v...
Embodiment 2V
[0095] Purification and identification of embodiment 2VSV recombinant virus
[0096] 1. Purification and identification of VSV recombinant virus
[0097] (1) Concentrate the amplified virus liquid VSV-△G-gB, VSV-△G-gB-G and VSV-△G-gHgL respectively in a high-speed centrifuge, and centrifuge at 100,000g for 2 hours at 4°C , and the pellet was resuspended in 1 mL of PBS.
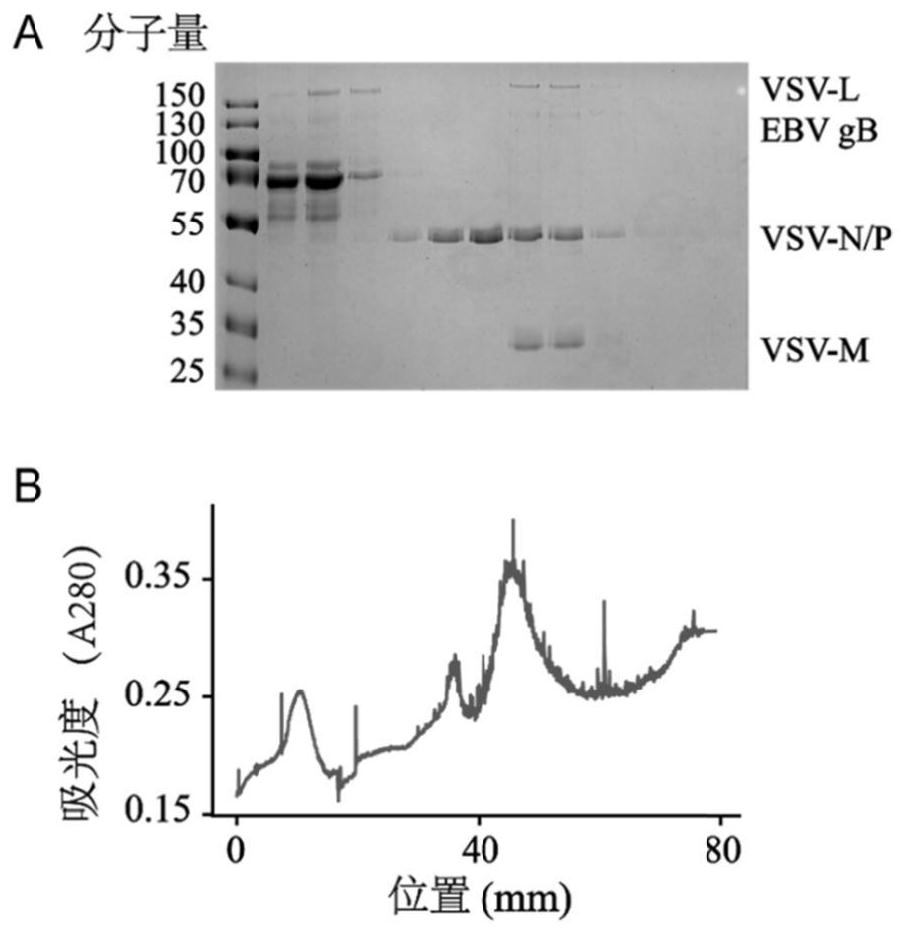
[0098] (2) Prepare a 20%-50% sucrose density gradient with a density gradient preparation apparatus, and ultracentrifuge the concentrated VSV-△G-gB and VSV-△G-gHgL virus liquids at 4°C at 40,000g for 16 hours , using a 20% to 50% sucrose density gradient for separation, you can see an obvious white band in the centrifuge tube, which is the virus sample layer.
[0099] (3) Take out the samples after ultracentrifugation in layers with a density gradient separator, take a small amount of samples respectively, and analyze the distribution of samples in each layer with Coomassie Brilliant Blue staining experiment...
Embodiment 3
[0103] Embodiment 3 mouse immunization
[0104] 1. VSV-ΔG-gB without adjuvant
[0105] 1) Take 40 Balb / c mice, start immunization at the age of 8 weeks, and give a booster immunization in the 3rd and 6th weeks after the initial immunization (see Figure 5 );
[0106] 2) Prepare 200 μL of the following vaccines and administer them by subcutaneous injection in the abdomen: VSV-ΔG-1E6, VSV-ΔG-gB-1E5, VSV-ΔG-gB-1E6, VSV-ΔG-gB-1E7, VSV-ΔG-gB -1E8;
[0107] 3) Before immunization, two weeks after the initial immunization, and two weeks after each booster immunization, blood was collected from the retrocanthus venous plexus, 5-6 drops each time, and collected into 1.5ml EP tubes;
[0108] 4) Incubate at 37°C for 30 minutes; centrifuge at 18,000 rcf at 4°C for 30 minutes;
[0109] 5) Transfer the supernatant to a new EP tube and inactivate at 56°C for 30 minutes;
[0110] 6) Store at -80°C for later use;
[0111] 2. VSV-ΔG-gB-G, with aluminum adjuvant
[0112] 1) Take 20 C57 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com