Patents
Literature
59 results about "Non neoplastic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-neoplastic means that a mass is not a tumor. It is reactive. For example, fibrocystic change in breasts, reactive lymphadenitis (lymph nodes reacting to infection), benign prostatic hypertrophy. Benign does not imply pathophysiology, only clinical behavior. A reactive lesion is, of course, benign, as is a non-invasive neoplasm.
Conjugates of rgd peptides and porphyrin or (bacterio)chlorophyll photosynthesizers and their uses
ActiveUS20120294801A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsPorphyrinWilms' tumor
Conjugates of porphyrin, chlorophyll and bacteriochlorophyll photosensitizers with RGD-containing peptides or RGD peptidomimetics are provided that are useful for photodynamic therapy (PDT), particularly vascular-targeted PDT (VTP), of tumors and nonneoplastic vascular diseases such as age-related macular degeneration, and for diagnosis of tumors by different techniques.
Owner:YEDA RES & DEV CO LTD
Methods for evaluating pathologic conditions using extracellular RNA
This invention provides methods for the detection, diagnosing, monitoring, or predicting of non-neoplastic diseases, pathologic conditions, and injury. The methods of the invention detect extracellular non-neoplastic mammalian RNA in the blood, blood plasma, serum, or other bodily fluid of an animal, most preferably a human, having or predisposed to having a non-neoplastic disease, pathologic condition, or injury.
Owner:ONCOMEDX
High fluence rate activation of photosensitizers for dermatological applications
Treatment of neoplastic or non-neoplastic dermatological conditions is achieved by administration and photodynamic activation of photosensitizers and pro-photosensitizers by coherent and / or incoherent light. The treatment does not cause clinically signification side effects such as purpura of the treated skin.
Owner:CANDELA CORP
Topographic genotyping for determining the diagnosis, malignant potential, and biologic behavior of pancreatic cysts and related conditions
InactiveUS20060088870A1Easy to analyzeEnhancing definitive diagnosisHealth-index calculationMicrobiological testing/measurementMolecular analysisMicrosatellite
The application relates to a method of a predicting the presence of invasive pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions comprising detailed molecular analysis incorporating DNA quality and quantity, K-ras mutational analysis and a broad spectrum of tumor suppressor gene linked microsatellite LOH. Methods of diagnosing, determining prognosis of and determining a course of treatment for pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions are also provided.
Owner:REDPATH INTEGRATED PATHOLOGY INC
Method for the Molecular Diagnosis of Prostate Cancer and Kit for Implementing Same
InactiveUS20100227317A1Microbiological testing/measurementImmunoglobulinsProstate sampleIn vitro analysis
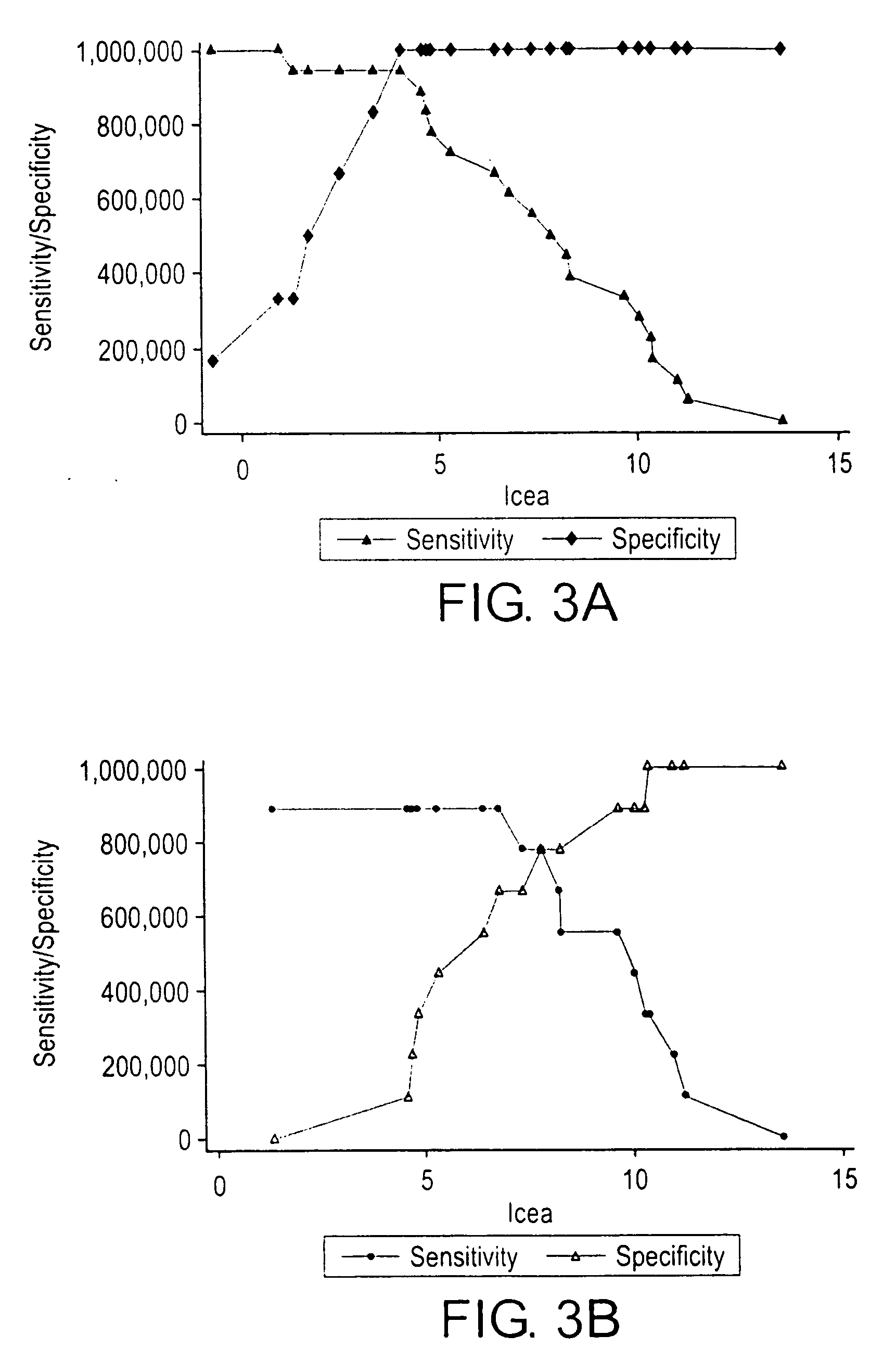
The invention relates to a method for the molecular diagnosis of prostate cancer, comprising the in vitro analysis of the overexpression or underexpression of combinations of genes that can distinguish, with high statistical significance, tumorous prostate samples from non-tumorous prostate samples. The invention also relates to a kit for the molecular diagnosis of prostate cancer, which can perform the above-mentioned detection.
Owner:FUNDACIO PRIVADA CLINIC PER A LA RECERCA BIOMEDICA +1
Use of regularly scheduled high dose intravenous methotrexate therapy, with interim administration of immunomodulatory agents, to treat multiple sclerosis and other diseases of the central nervous system
InactiveUS6903100B2Halted deteriorationImprove scoreBiocidePeptide/protein ingredientsCytotoxicityHigh doses
The present invention is directed to the treatment of multiple sclerosis by periodically administering a high dose of methotrexate at a level sufficiently high to cross the blood brain barrier. The methotrexate administration is accompanied by leucovorin rescue of the periphery. The high dose methotrexate is preferably administered at 1 to 4 month intervals. The periodic high dose methotrexate treatment may be used in conjunction with interim treatments using a therapeutic agent that is effective in treating MS, but does not cross the BBB in cytotoxic amounts. It is contemplated that the method of the present invention may be employed to treat other non-infectious, non-neoplastic inflammatory conditions of the CNS.
Owner:MIDAMERICA NEUROSCI RES FOUND
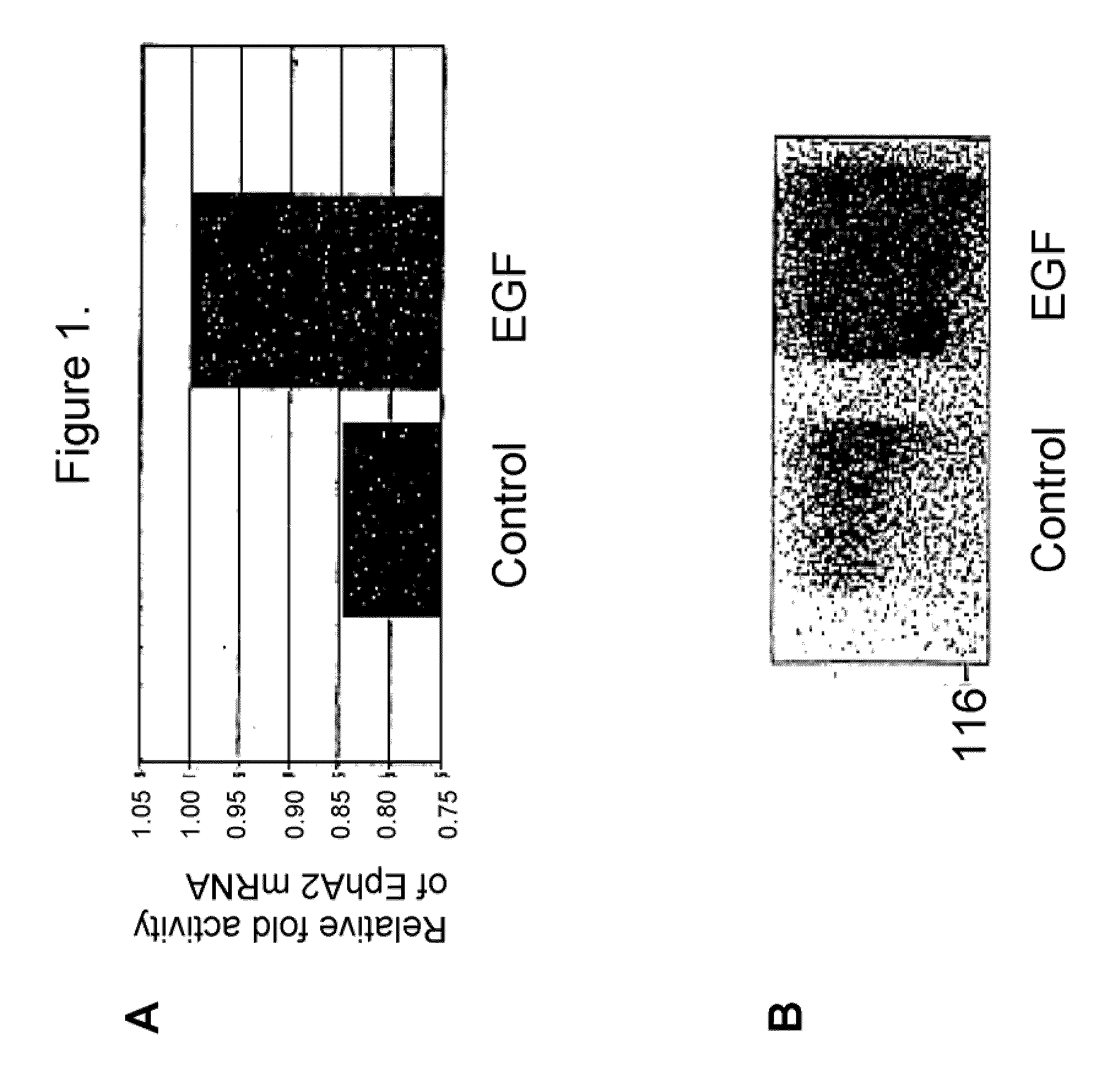
Modulators of EphA2 and EphrinA1 for the treatment of fibrosis-related disease
InactiveUS20060122138A1Maintaining organizationInhibit and decrease angiogenesisSenses disorderAntipyreticPsoriatic arthropathyDiabetic retinopathy
The present invention relates to methods and compositions designed for the treatment, management, prevention and / or amelioration of non-neoplastic hyperproliferative epithelial and / or endothelial cell disorders, including but not limited to disorders associated with increased deposition of extracellular matrix components (e.g., collagen, proteoglycans, tenascin and fibronectin) and / or aberrant angiogenesis. Non-limiting examples of such disorders include cirrhosis, fibrosis (e.g., fibrosis of the liver, kidney, lungs, heart, retina and other viscera), asthma, ischemia, atherosclerosis, diabetic retinopathy, retinopathy of prematurity, vascular restenosis, macular degeneration, rheumatoid arthritis, osteoarthritis, infantile hemangioma, verruca vulgaris, Kaposi's sarcoma, neurofibromatosis, recessive dystrophic epidermolysis bullosa, ankylosing spondylitis, systemic lupus, Reiter's syndrome, Sjogren's syndrome, endometriosis, preeclampsia, atherosclerosis, coronary artery disease, psoriatic arthropathy and psoriasis. The methods of the invention comprise the administration of an effective amount of one or more agents that are modulators of EphA2 and / or its endogenous ligand, EphrinA1. The invention also provides pharmaceutical compositions comprising one or more EphA2 / EphrinA1 Modulators of the invention either alone or in combination with one or more other agents useful for therapy for such non-neoplastic hyperproliferative epithelial and / or endothelial disorders. Diagnostic methods and methods for screening for EphA2 / EphrinA1 Modulators are also provided.
Owner:MEDIMMUNE LLC
Compositions and methods for protecting cells during cancer chemotherapy and radiotherapy
InactiveUS7531562B2Protection from damagePrevent baldnessBiocideHeavy metal active ingredientsRadical radiotherapyChemoprotection
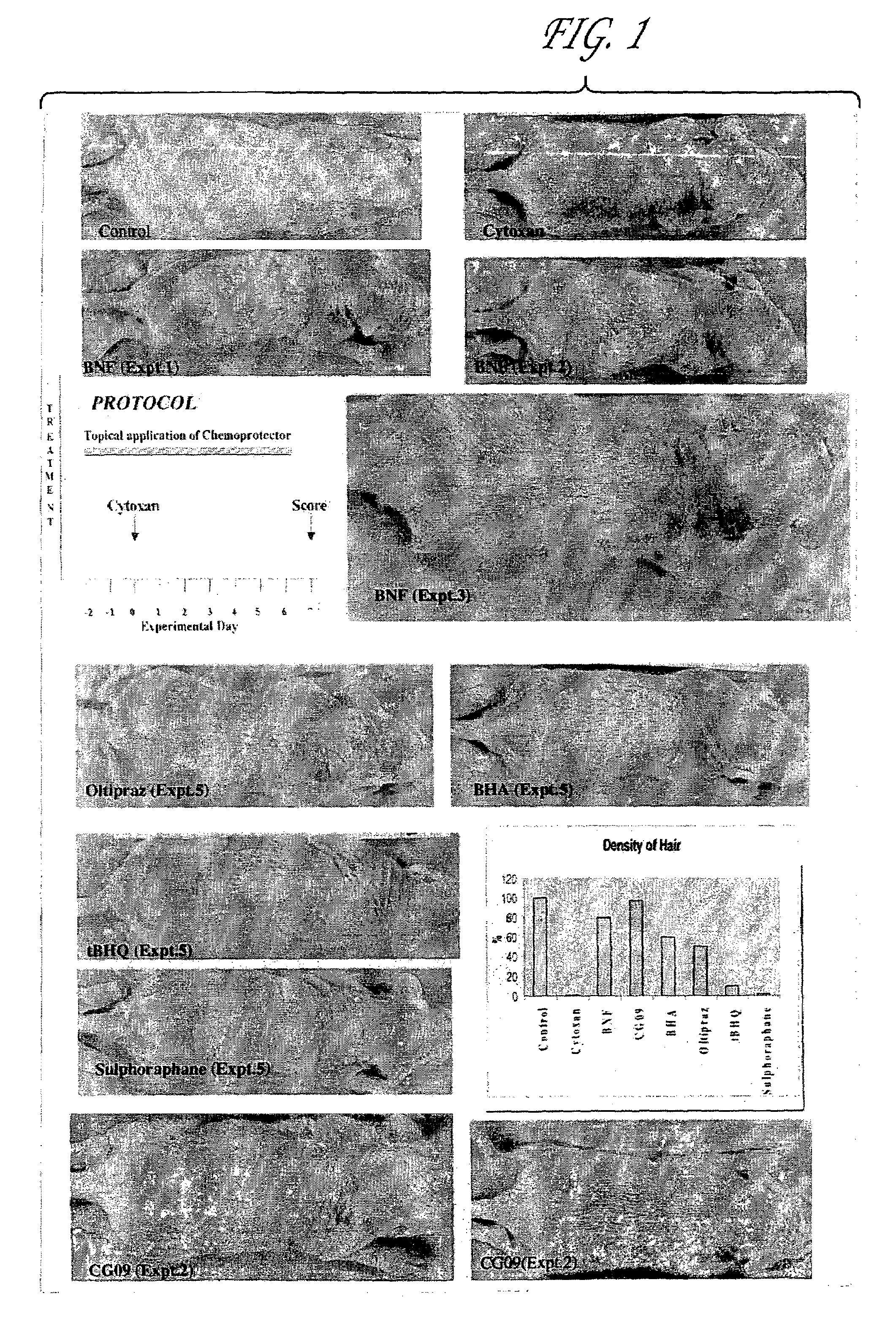
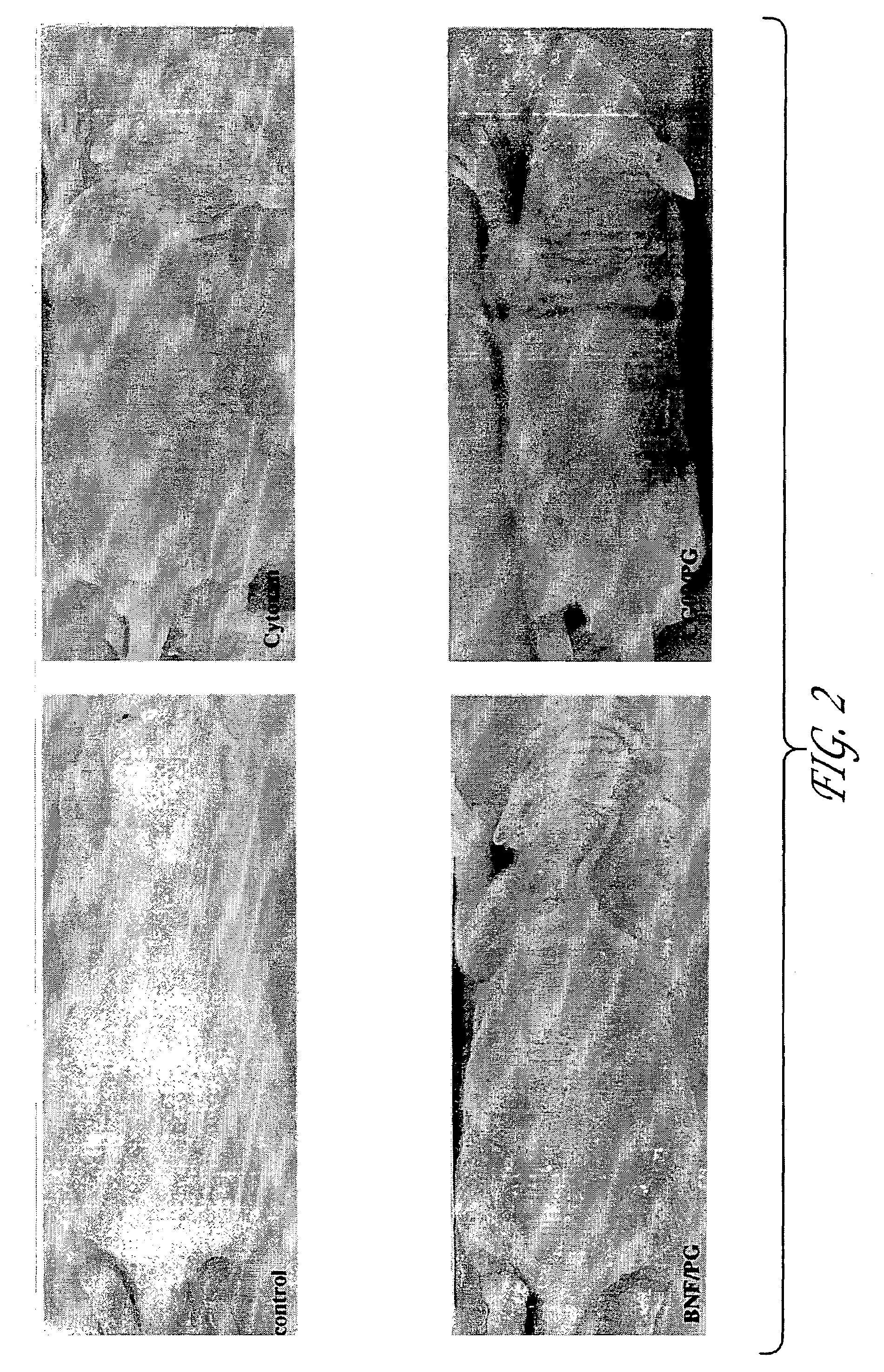
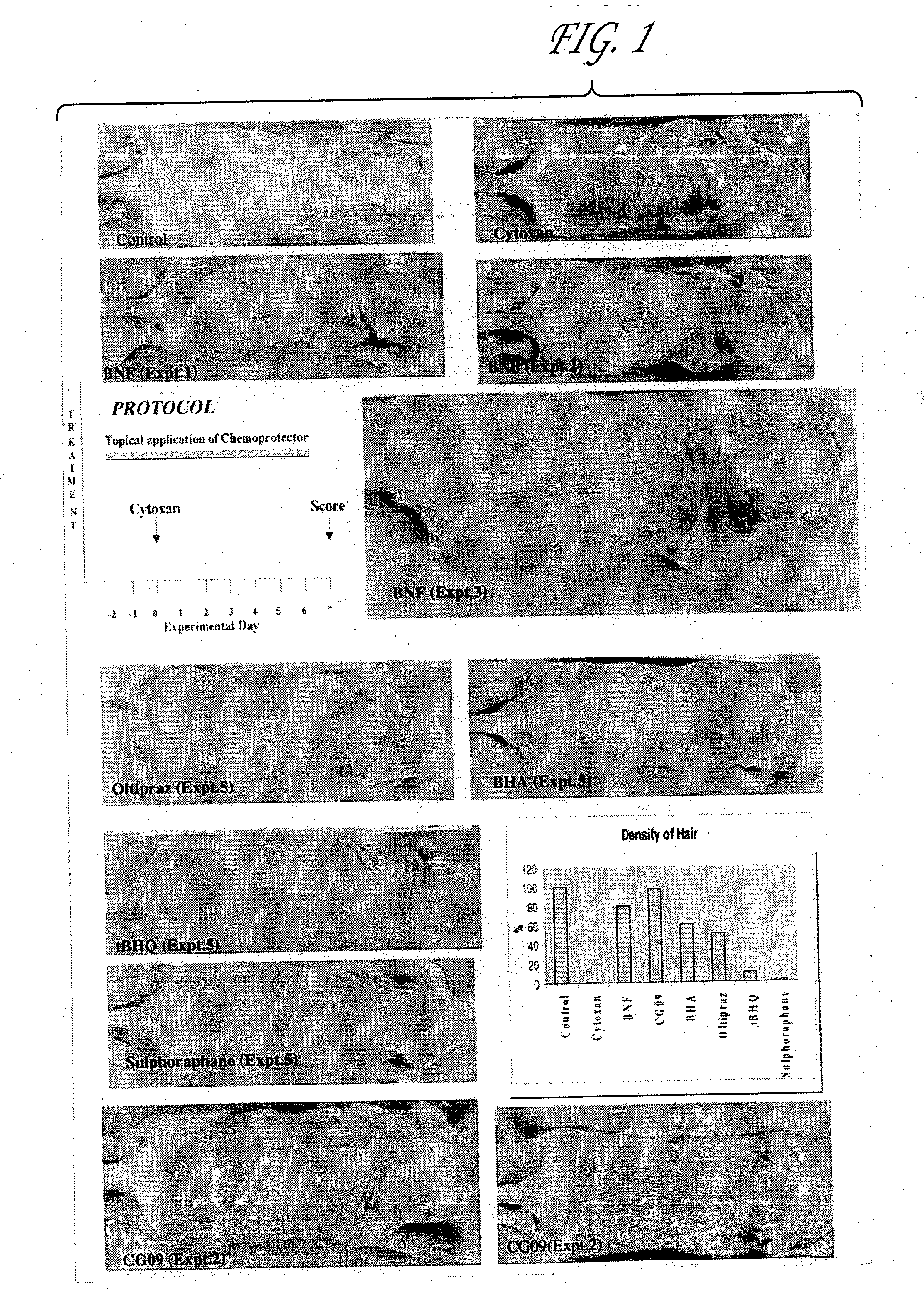
Compositions, pharmaceutical preparations and methods are disclosed for protecting non-neoplastic cells from damage caused by cancer chemotherapeutic agents or radiation therapy, during the course of cancer therapy or bone marrow transplant. These are based on the use of chemoprotective inducing agents that induce or increase production of cellular detoxification enzymes in target cell populations. The compositions and methods are useful to reduce or prevent hair loss, gastrointestinal distress and lesions of the skin and oral mucosa that commonly occur in patients undergoing cancer therapy. Also disclosed is a novel assay system for identifying new chemoprotective inducing agents.
Owner:WISCONSIN ALUMNI RES FOUND
Kits for treatment of hematologic disorders
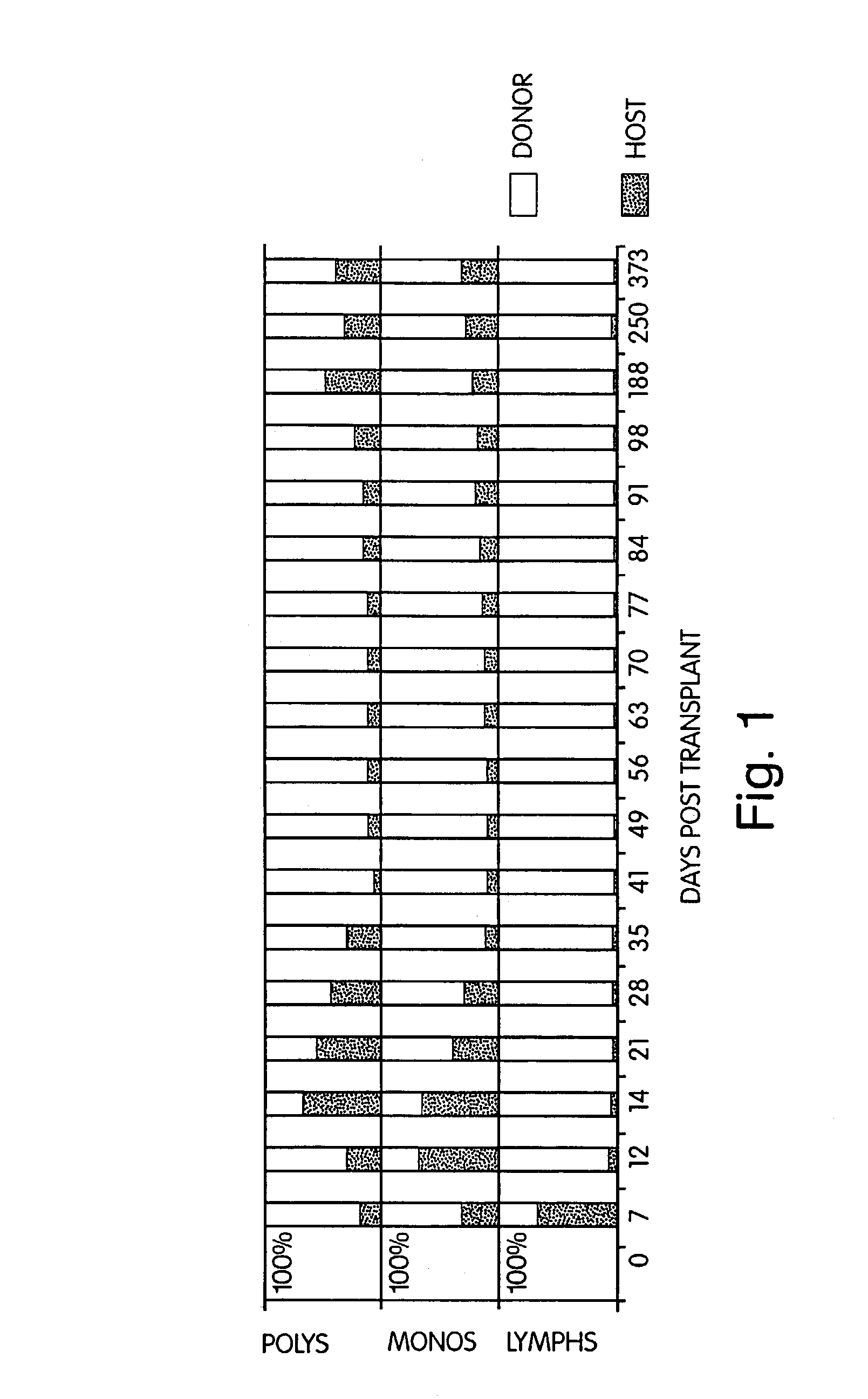
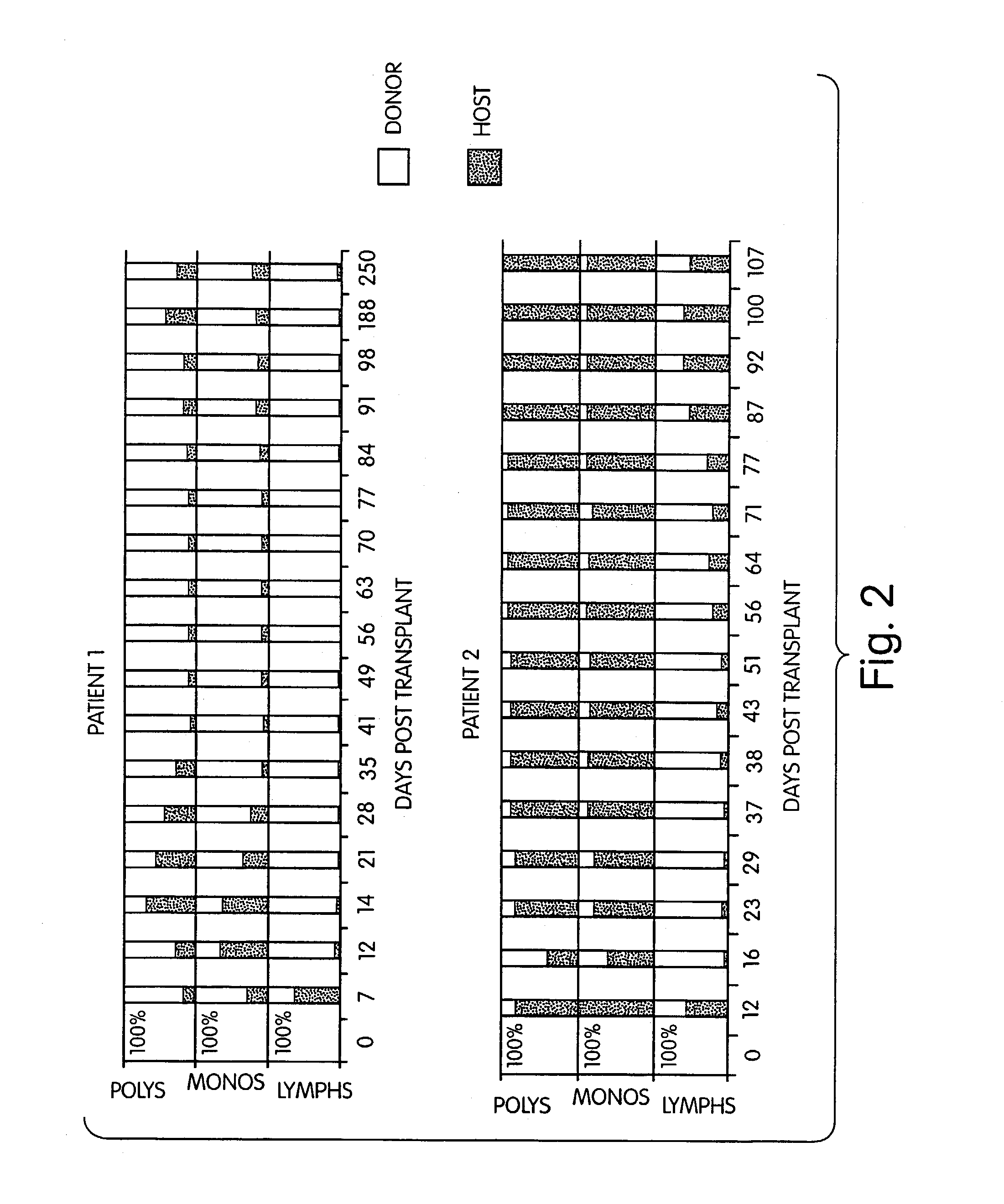
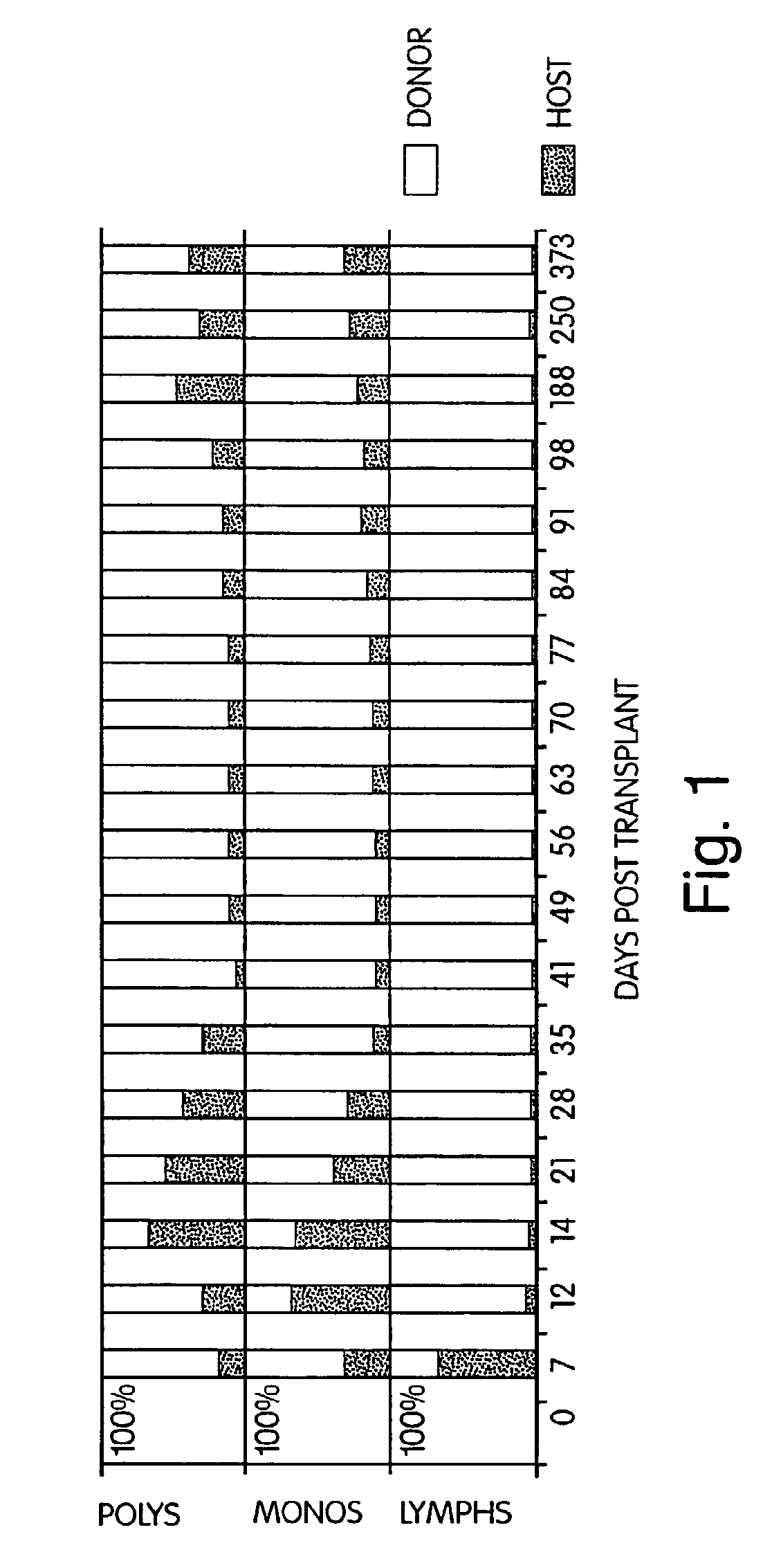
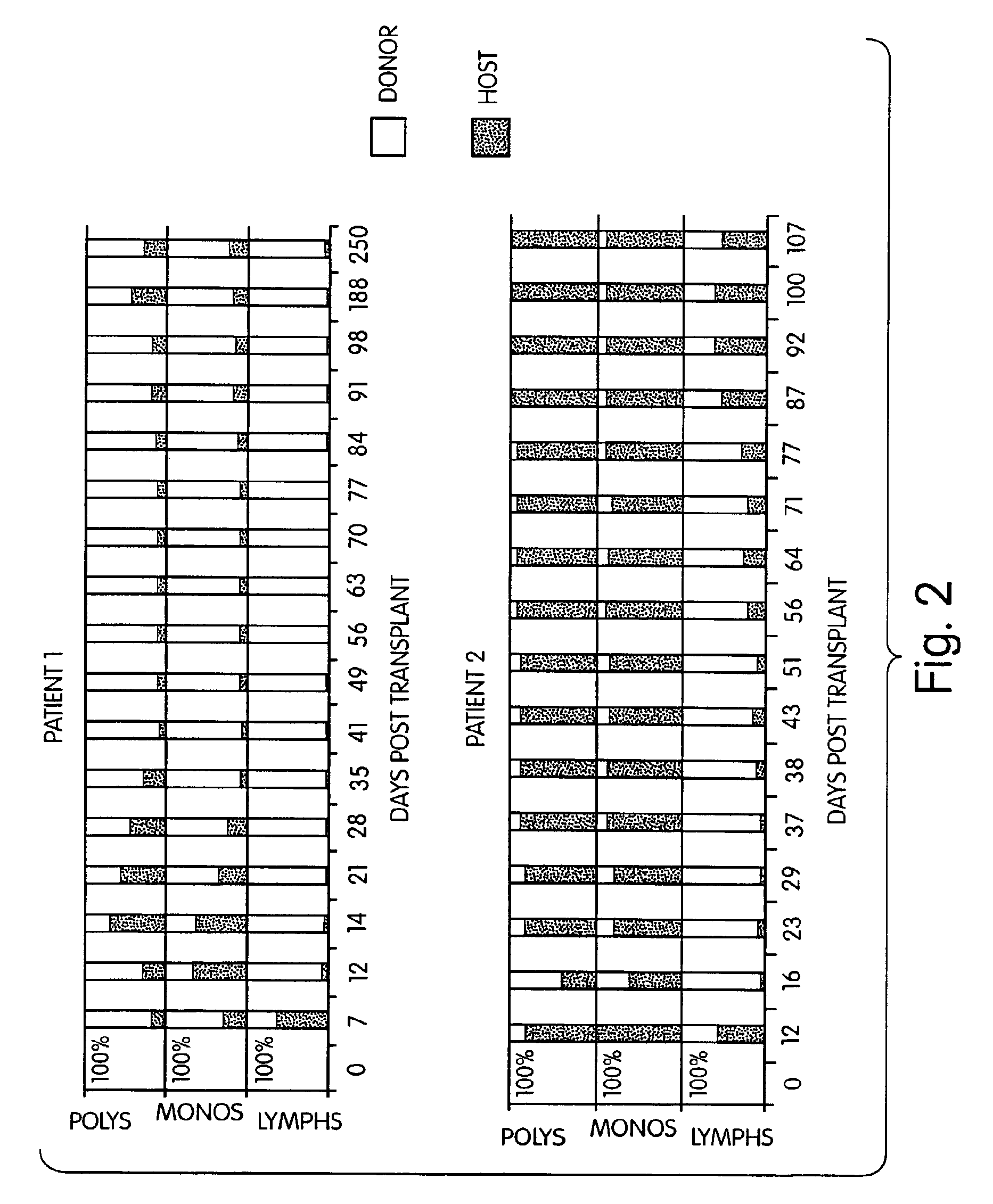
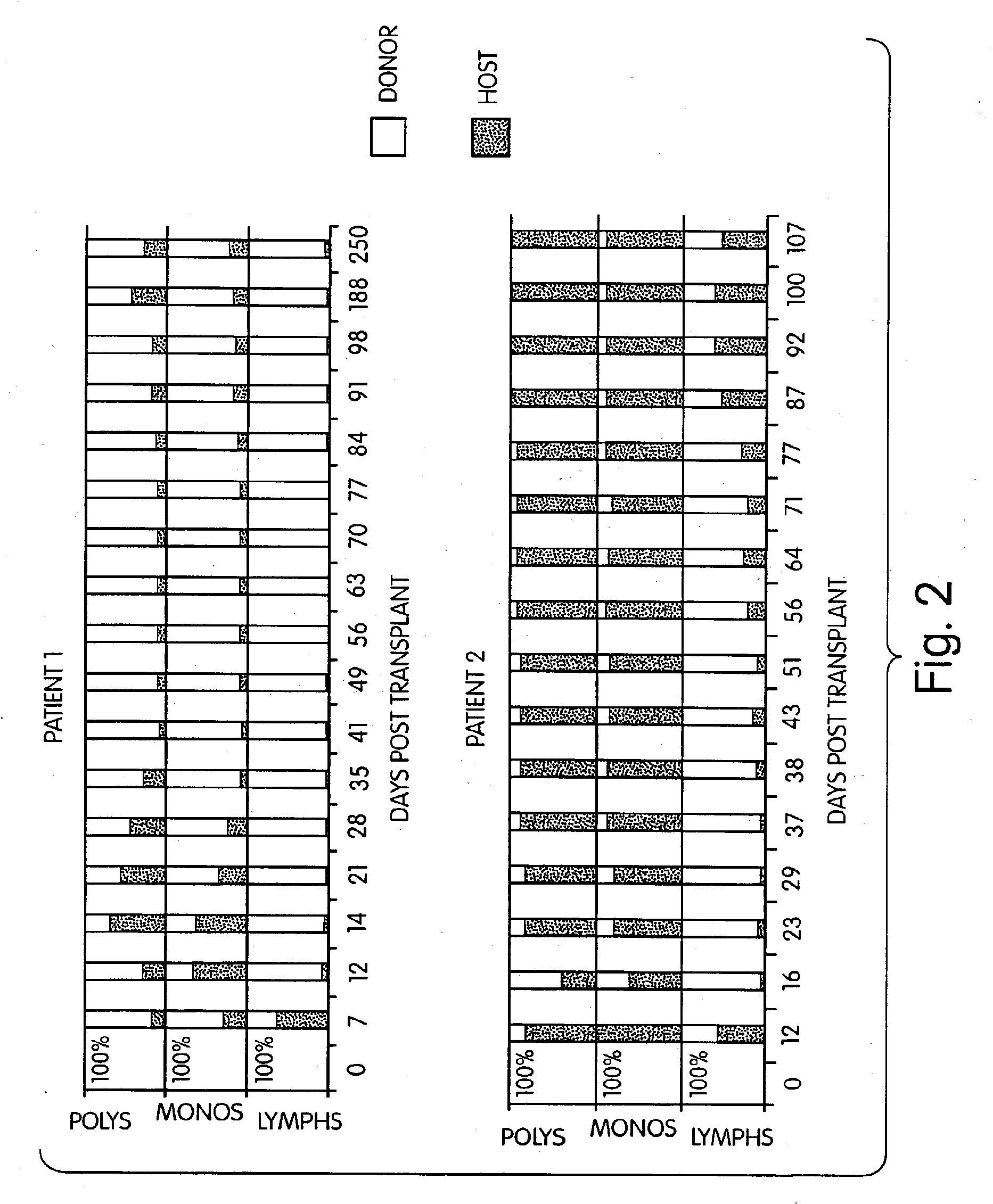
InactiveUS7408039B2Reduce GVHDSignificant graft-versus-leukemia (GVL) effectBiocideGenetic material ingredientsGraft versus leukaemiaHematological Diseases
The inventors have discovered that hematologic disorders, e.g., both neoplastic (hematologic cancers) and non-neoplastic conditions, can be treated by the induction of mixed chimerism using myeloreductive, but not myeloablative, conditioning. Methods of the invention reduce GVHD, especially GVHD associated with mismatched allogeneic or xenogeneic donor tissue, yet provide, for example, significant graft-versus-leukemia (GVL) effect and the like.
Owner:THE GENERAL HOSPITAL CORP
Treatment of hematologic disorders
InactiveUS7892578B2Reduce GVHDRemarkable effectBiocideOrganic active ingredientsGraft versus leukaemiaHematological Diseases
The inventors have discovered that hematologic disorders, e.g., both neoplastic (hematologic cancers) and non-neoplastic conditions, can be treated by the induction of mixed chimerism using myeloreductive, but not myeloablative, conditioning. Methods of the invention reduce GVHD, especially GVHD associated with mismatched allogeneic or xenogeneic donor tissue, yet provide, for example, significant graft-versus-leukemia (GVL) effect and the like.
Owner:THE GENERAL HOSPITAL CORP
Retinoids and use thereof
ActiveUS8475775B1Treating and preventing and ameliorating disorderAvoid adjustmentBiocideCosmetic preparationsRetinoidMedicine
The present invention provides new retinoid compounds and uses of the compounds in humans and animals for non-neoplastic dermal or inflammatory conditions or disorders.
Owner:UAB RES FOUND
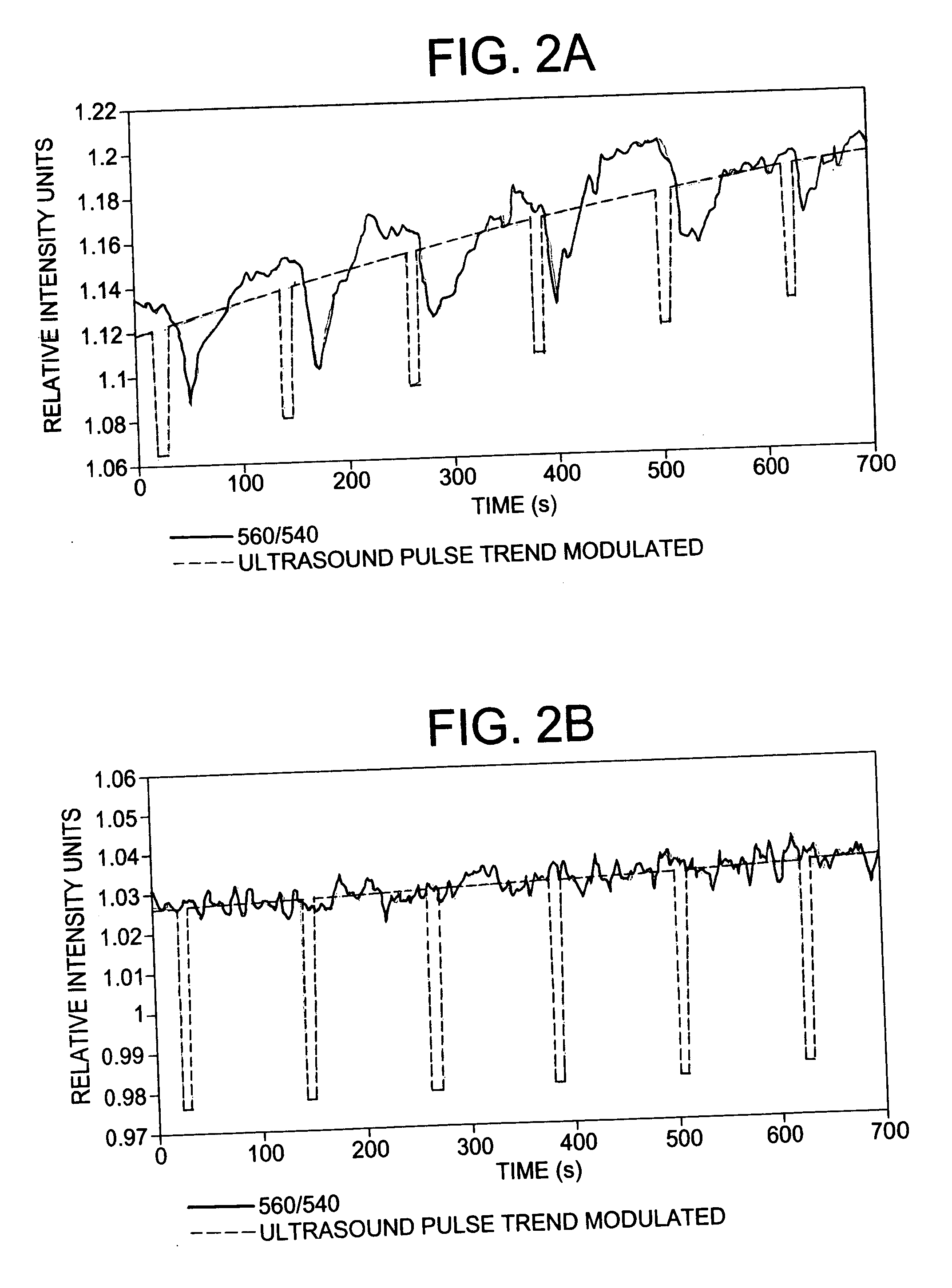
Acoustically induced blood stasis and in vivo optical spectroscopy
ActiveUS20070055126A1Avoid blood flowSusceptible to radiation damageUltrasound therapyDiagnostics using spectroscopyUltrasound imagingHealth risk
Ultrasound-induced blood stasis has been observed for more than thirty years. Most of the literature has been focused on the health risks associated with this phenomenon and methods employed to prevent stasis from occurring during ultrasound imaging. To date, experimental observations have been either in vitro or invasive. The current work demonstrates ultrasound-induced blood stasis in murine tumor and nontumor tissue, observed through noninvasive measurements of optical spectroscopy, and discusses possible diagnostic uses for this previously undesirable effect of ultrasound.
Owner:UNIVERSITY OF ROCHESTER
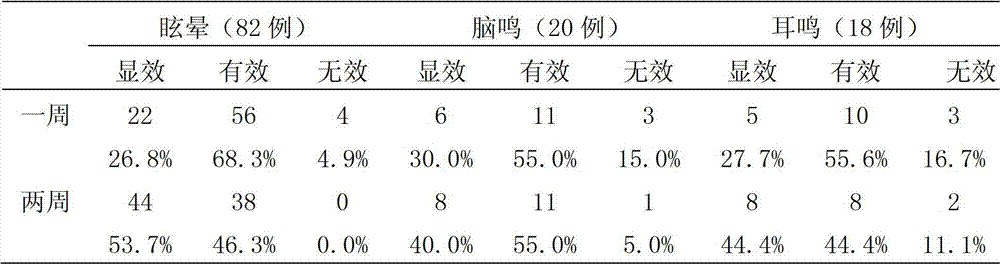
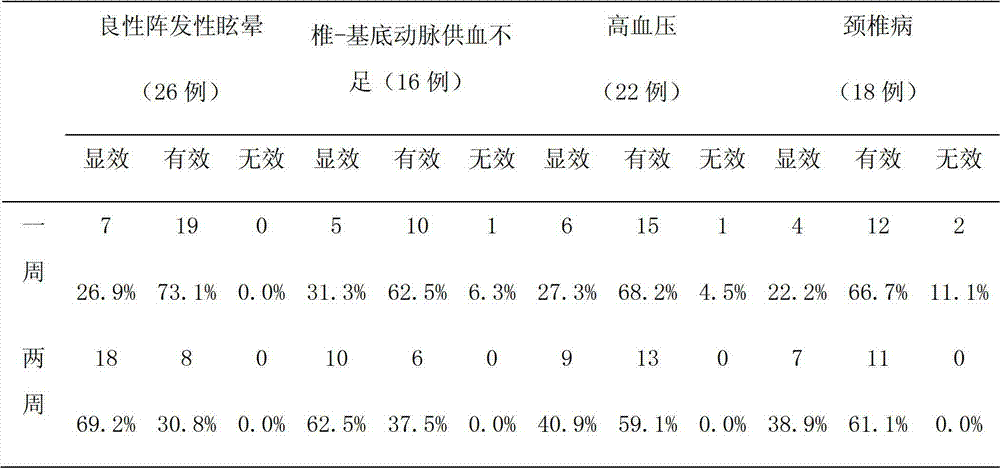
Traditional Chinese herbal composition for treating vertigo, preparation and method for preparing traditional Chinese herbal composition
InactiveCN102727689AEffective treatmentCertain curative effectDigestive systemPlant ingredientsLiver and kidneyCervical spondylosis
The invention discloses a traditional Chinese herbal composition for treating vertigo, which can effectively treat the symptoms of vertigo, aturdimiento, fullness in head, accompanied or non-accompanied headache, tinnitus, nausea and vomiting and the like, and is particularly suitable for the old. The traditional Chinese herbal composition is effective to subjective tinnitus which is caused by non-otogenic and organic lesions, non-neoplastic lesions and non-trauma. The traditional Chinese herbal composition has an exact effect on benign paroxysmal vertigo, a special curative effect on tinnitus cerebri, and obvious effects on vertigo which is caused by tinnitus, cerebral arteriosclerosis, cervical spondylosis and other factors, and is particularly suitable for vertigo which is caused by deficiency of liver and kidney, wind-phlegm and blood stasis and obstruction of meridians.
Owner:赵东
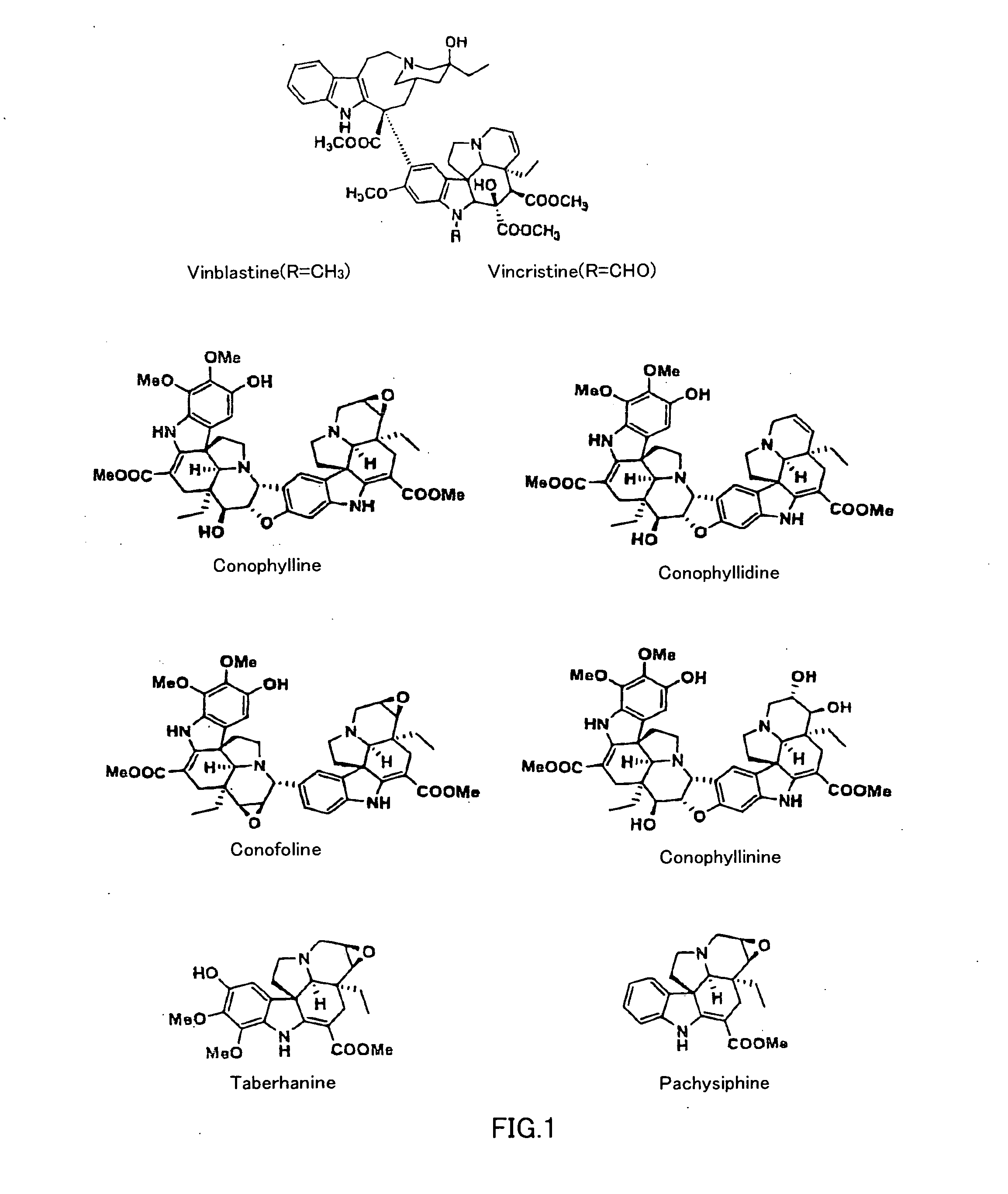
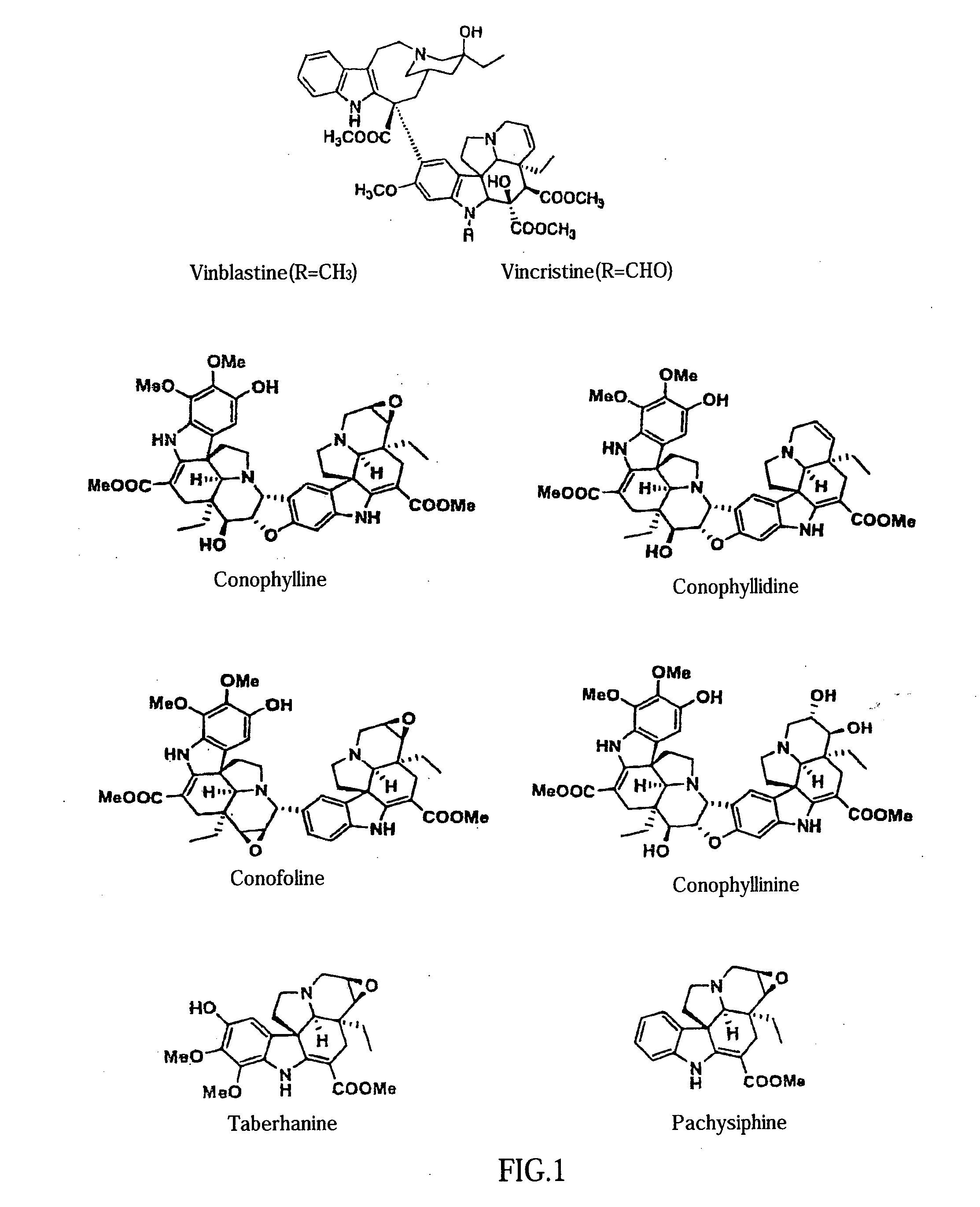
Use of Vinca Alkaloids and Salts Thereof
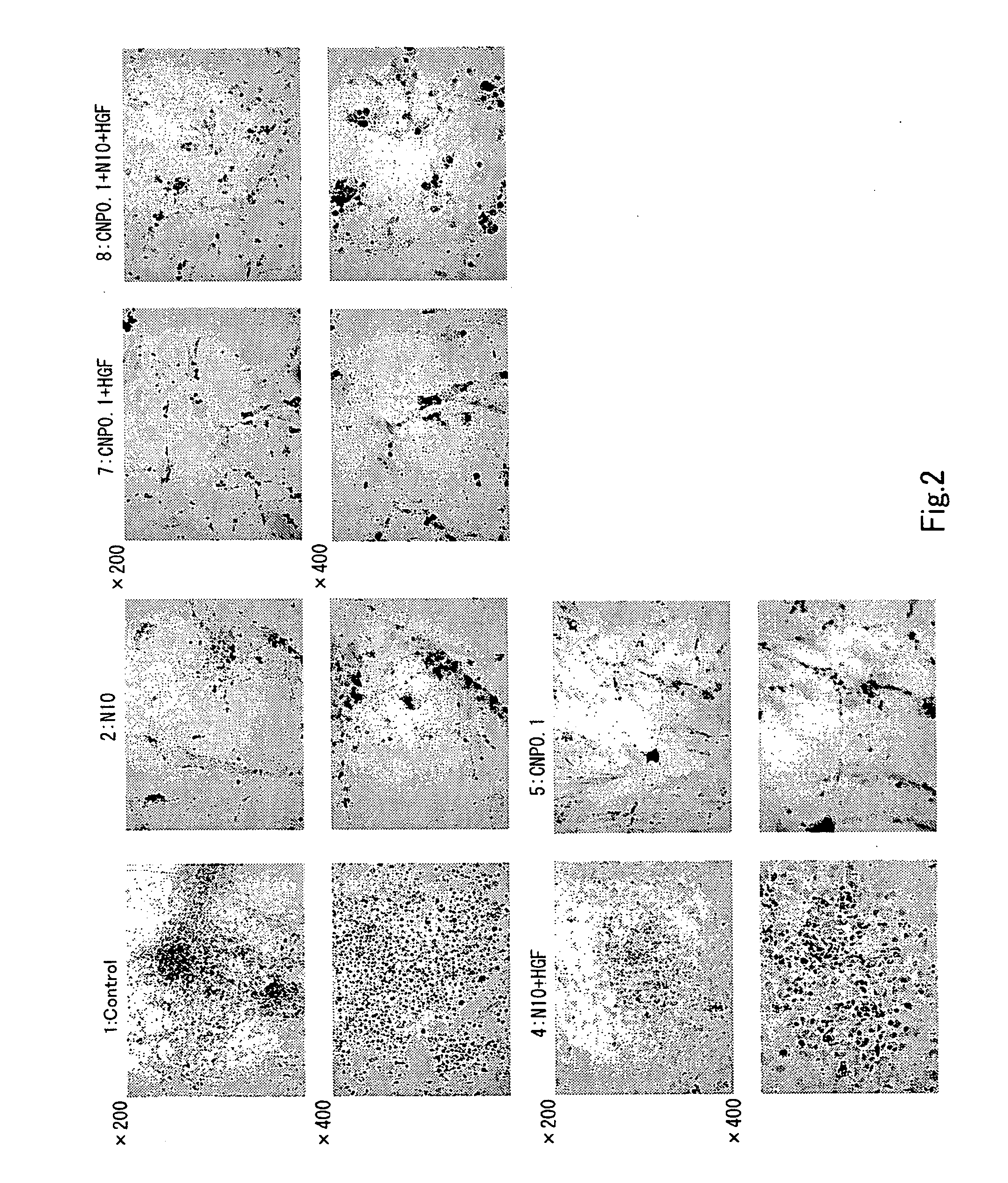
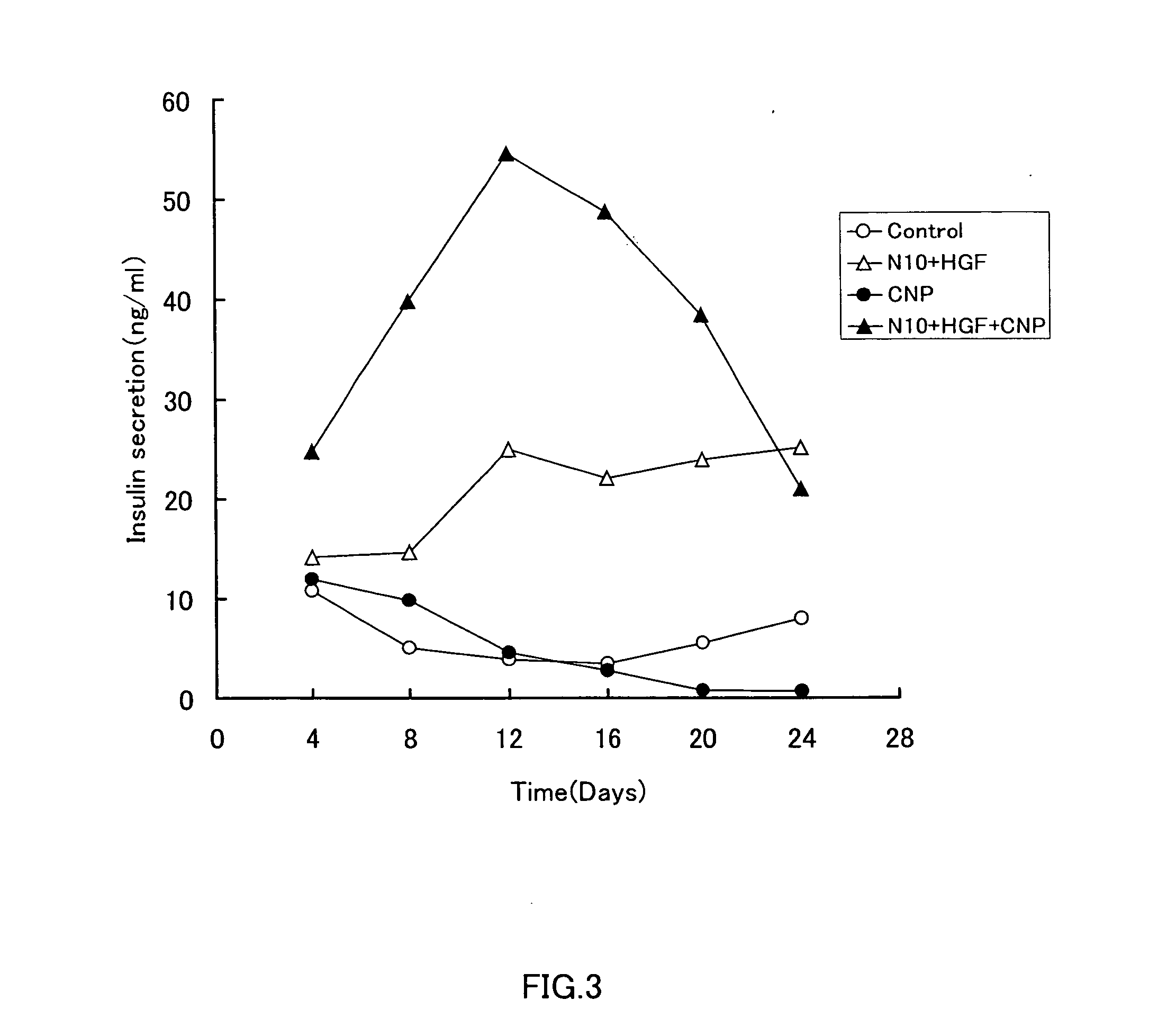
InactiveUS20070232533A1Increase insulin productionIncreasing insulin-producingBiocideSenses disorderMedicinePancreas
An agent containing a vinca alkaloid or its pharmacologically acceptable salt as an active ingredient can induce insulin production and / or secretion of non-neoplastic cells derived from the pancreas.
Owner:KEIO UNIV
Prevention of ovarian cancer by administration of a Vitamin D compound
InactiveUS7053074B2Avoid developmentIncrease ratingsBiocideAnimal repellantsApoptosisRectal epithelium
The present invention relates to methods for preventing the development of epithelial ovarian cancer by administering a Vitamin D compound in an amount capable of increasing apoptosis in non-neoplastic ovarian epithelial cells of the female subject.
Owner:NEW LIFE PHARMA
Modulators of EphA2 and Ephrin-A1 for the treatment of fibrosis-related disease
InactiveUS20100260749A1Promote growthIncreased proliferationOrganic active ingredientsSenses disorderDiseasePsoriatic arthropathy
The present invention relates to methods and compositions designed for the treatment, management, prevention and / or amelioration of non-neoplastic hyperproliferative epithelial and / or endothelial cell disorders, including but not limited to disorders associated with increased deposition of extracellular matrix components (e.g., collagen, proteoglycans, tenascin and fibronectin) and / or aberrant angiogenesis. Non-limiting examples of such disorders include cirrhosis, fibrosis (e.g., fibrosis of the liver, kidney, lungs, heart, retina and other viscera), asthma, ischemia, atherosclerosis, diabetic retinopathy, retinopathy of prematurity, vascular restenosis, macular degeneration, rheumatoid arthritis, osteoarthritis, infantile hemangioma, verruca vulgaris, Kaposi's sarcoma, neurofibromatosis, recessive dystrophic epidermolysis bullosa, ankylosing spondylitis, systemic lupus, Reiter's syndrome, Sjogren's syndrome, endometriosis, preeclampsia, atherosclerosis, coronary artery disease, psoriatic arthropathy and psoriasis. The methods of the invention comprise the administration of an effective amount of one or more agents that are modulators of EphA2 and / or its endogenous ligand, EphrinA1. The invention also provides pharmaceutical compositions comprising one or more EphA2 / EphrinA1 Modulators of the invention either alone or in combination with one or more other agents useful for therapy for such non-neoplastic hyperproliferative epithelial and / or endothelial disorders. Diagnostic methods and methods for screening for EphA2 / EphrinA1 Modulators are also provided.
Owner:MEDIMMUNE LLC
Cell-specific signaling biomarker analysis by high parameter cytometry; sample processing, assay set-up, method, analysis
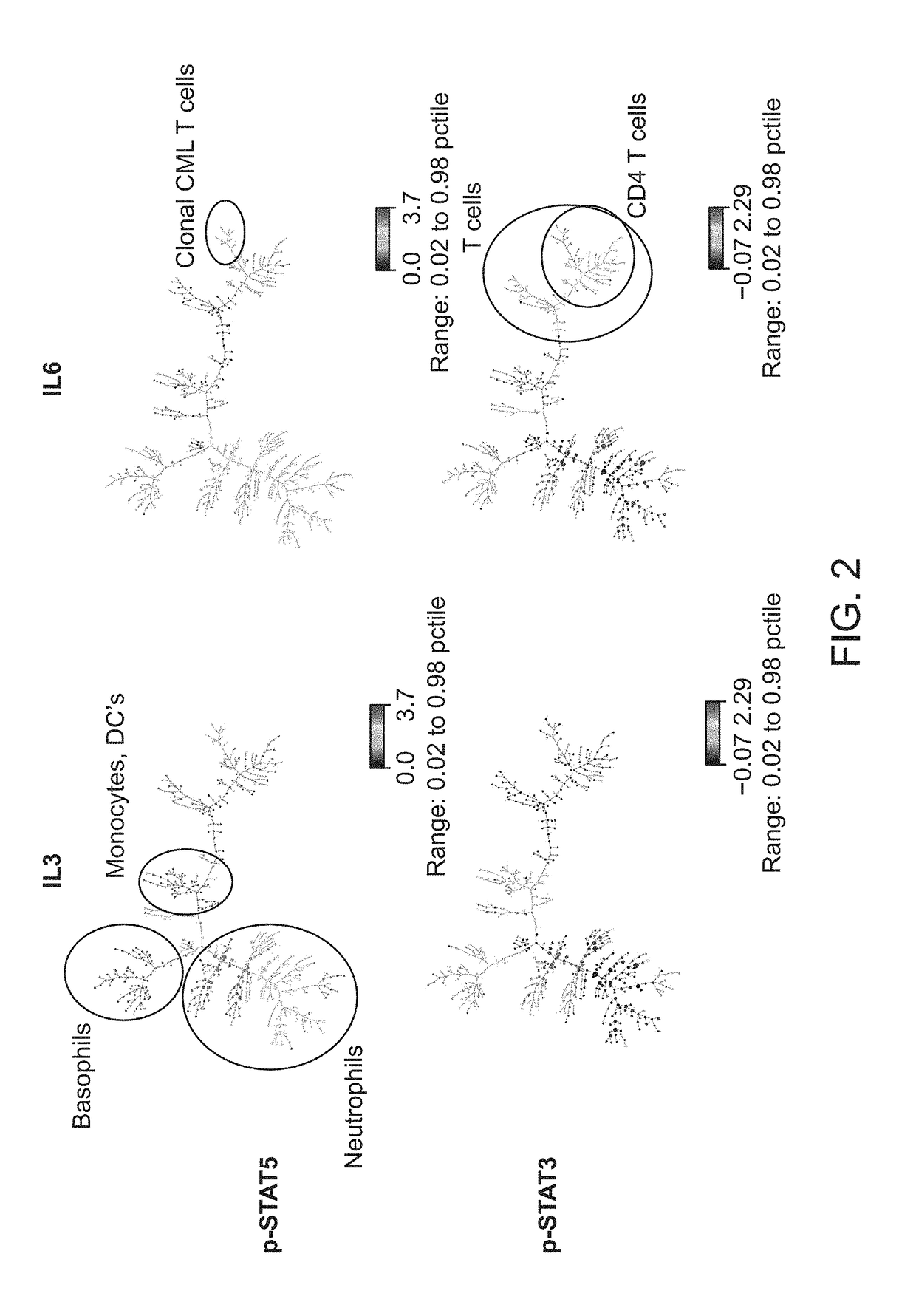
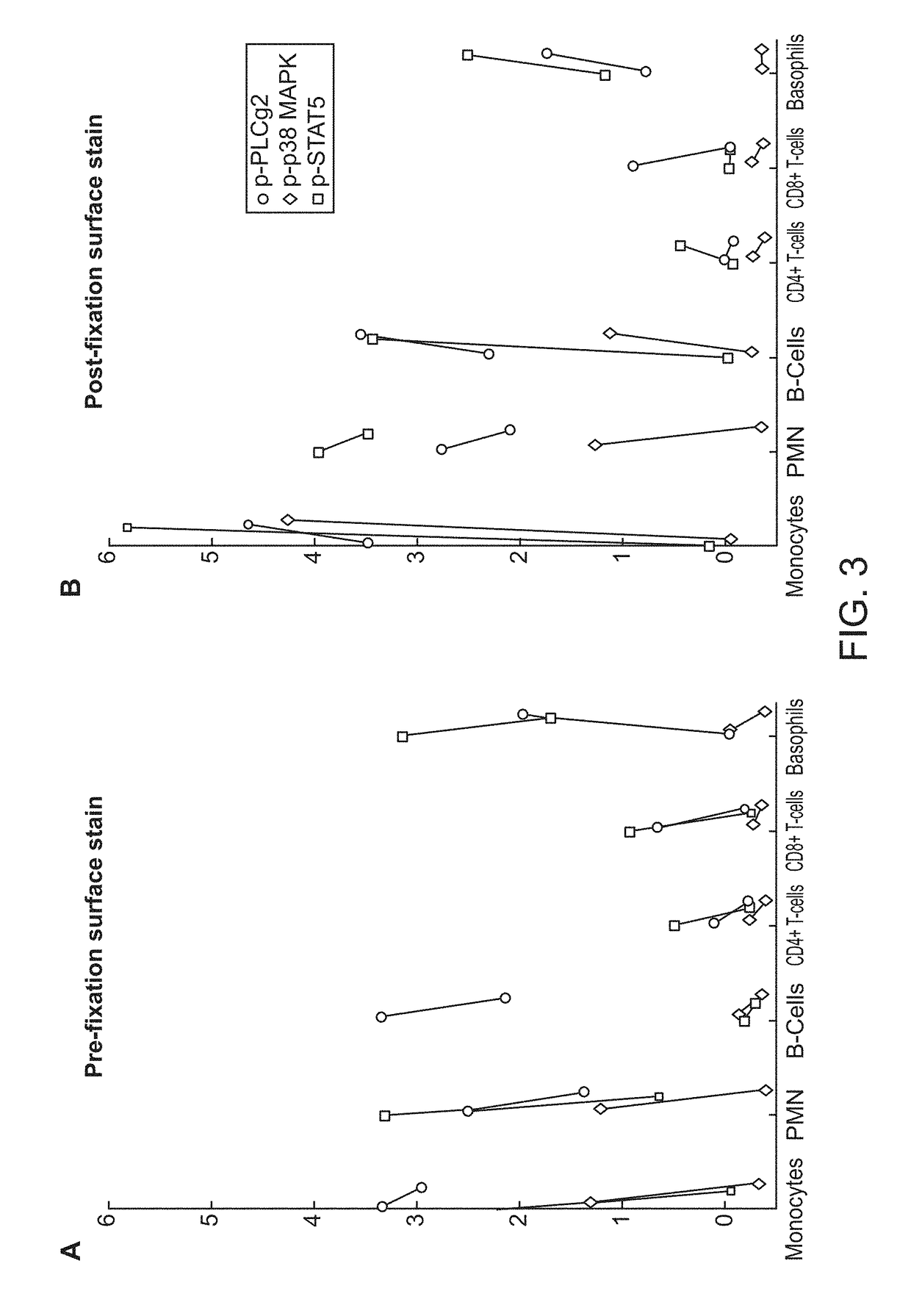
ActiveUS20180252708A1Facilitate data interpretationEasy to explainDisease diagnosisIndividual particle analysisMarker selectionProgenitor cell
The present invention recognizes that current clinical laboratory testing methods for multiparametric single cell analysis are limited to analysis of intact live cells, and are insufficient for identification of signaling activation profile defining certain cell types, including but not limited to neoplastic and immunologically activated cell subsets. One aspect of the present invention generally relates to marker selection in panels to include proteins routinely assessed in standard FCM, while preferably also incorporating markers for surface receptor proteins within activated signaling cascades. A further aspect of the present invention generally relates to panel design for the following indications in neoplastic and non-neoplastic clinical applications as examples of the technology: (a) identification of CML progenitor cell subsets in the setting of disease recurrence after treatment discontinuation or relapse due to treatment resistance, and (b) characterization of activated basophils to predict the severity of an allergic response. Another aspect of the present invention generally relates to methods to measure levels of surface and IC biomarkers in separate or combined assays for robust characterization of each or select cell compartment, and data analysis based on results from each or all method(s) used for optimal detection of the markers. A further aspect of the present invention generally relates to the identification and profiling of cell subpopulations based on analysis of surface markers including those associated with lineage and maturation of cell types and receptor proteins, and the corresponding IC phosphoproteins including those in activated signaling cascades to predict certain disease states or response to treatment.
Owner:DEEPATH MEDICAL
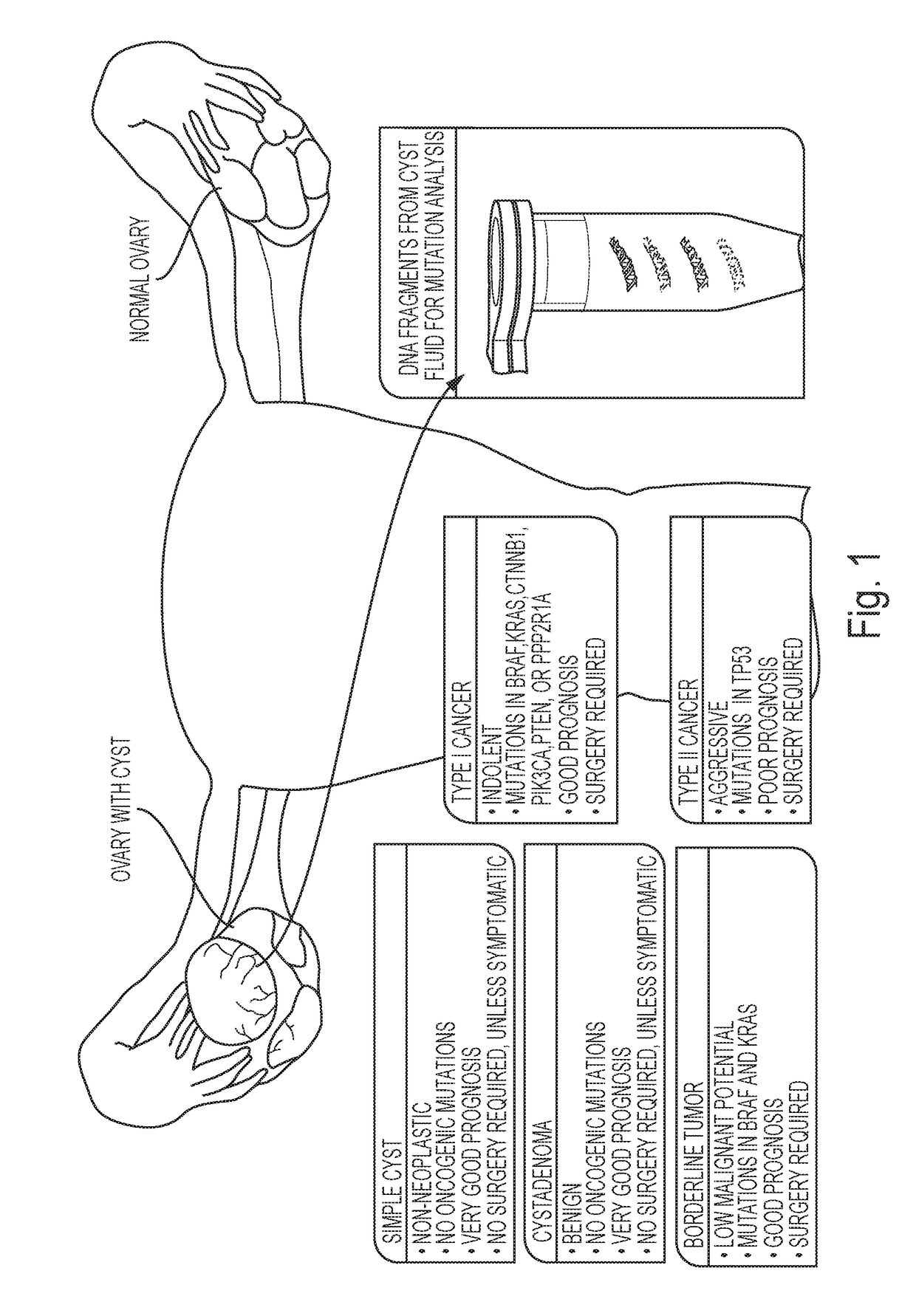
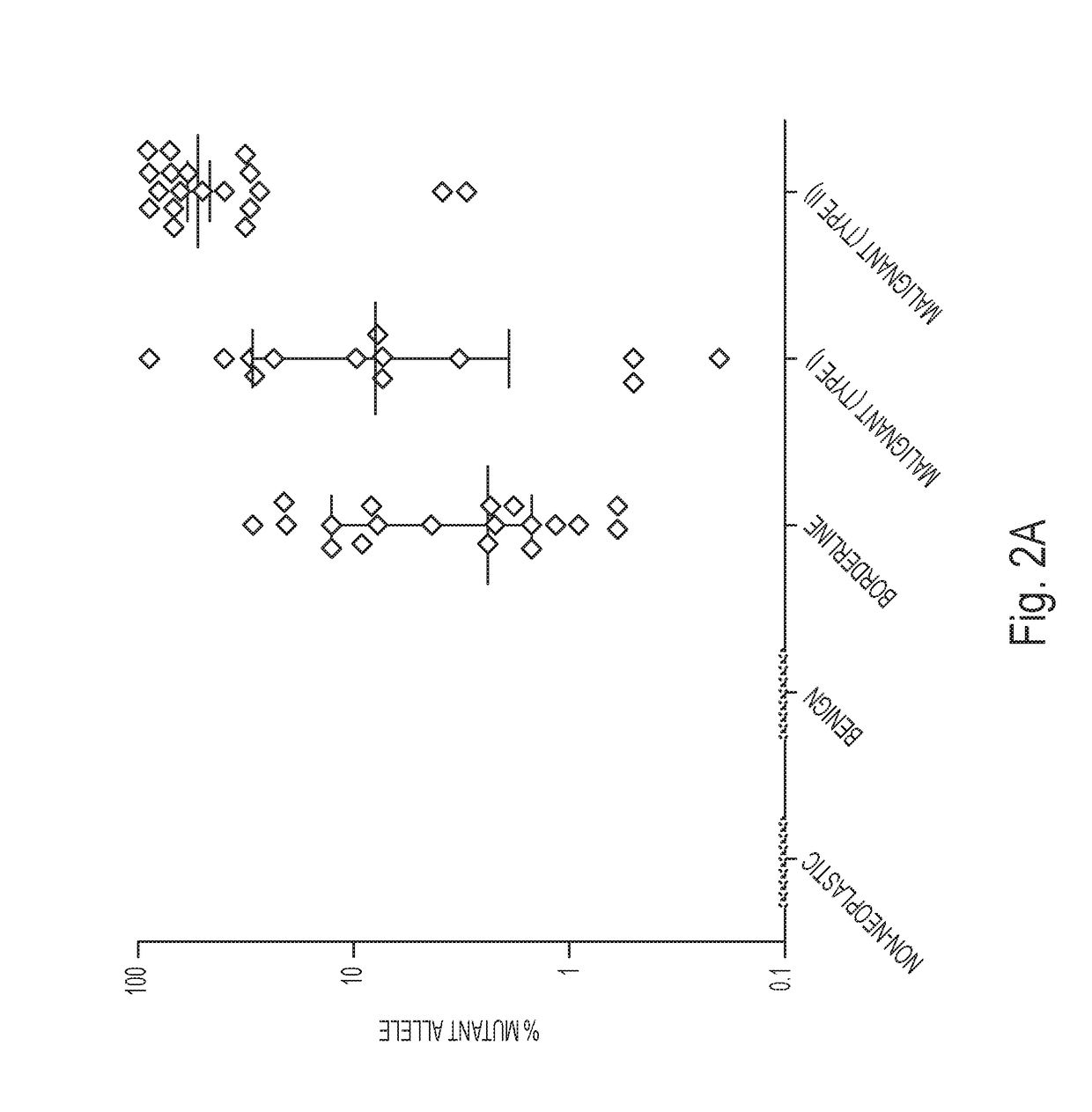
Assaying ovarian cyst fluid
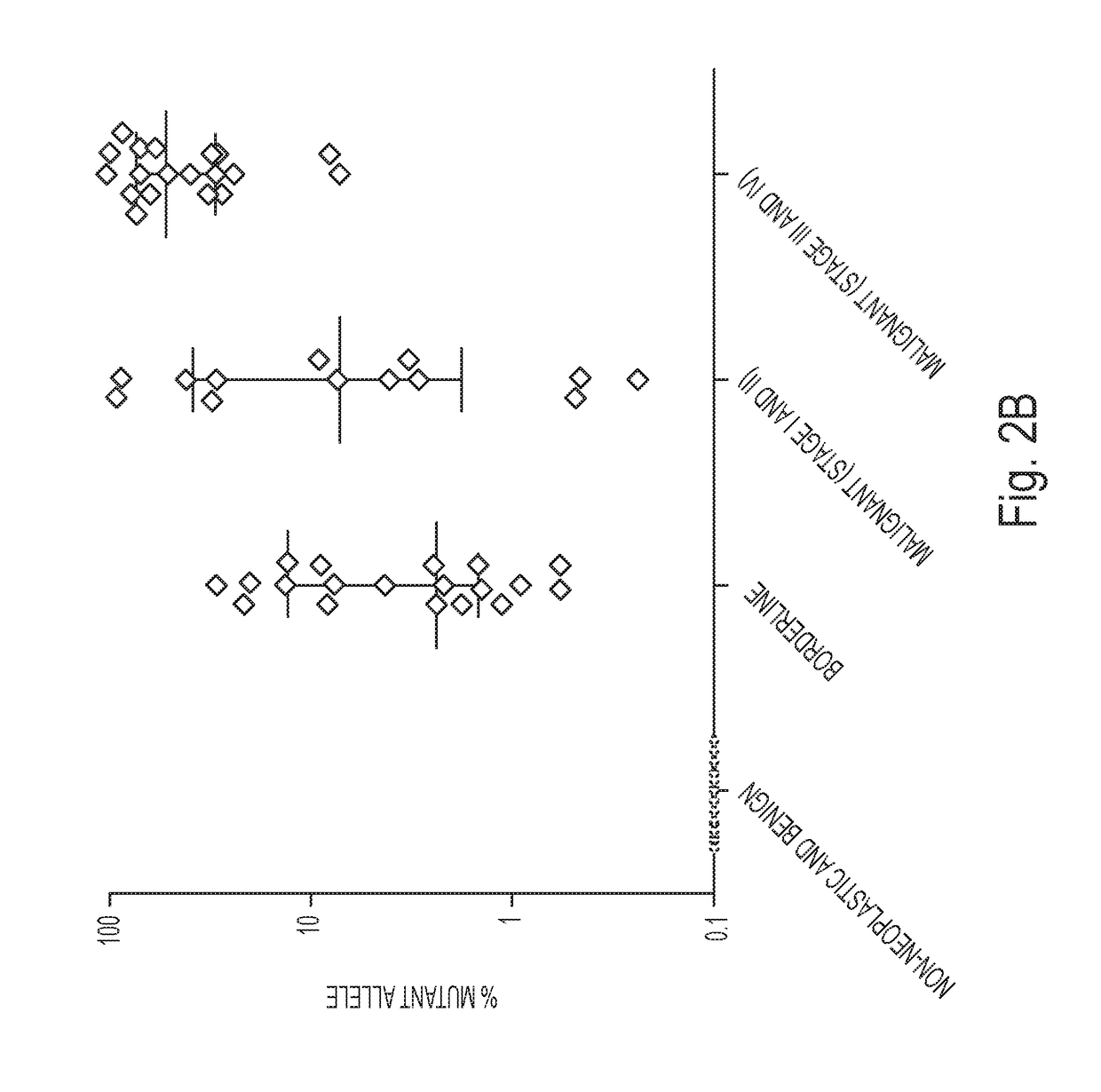
A diagnostic test for ovarian cysts is based on the detection of mutations characteristic of the most common neoplasms giving rise to these lesions. With this test, tumor-specific mutations were detected in the cyst fluids of 19 of 24 (79%) borderline tumors and 28 of 31 (90%) malignant ovarian cancers. In contrast, we detected no mutations in the cyst fluids from 10 non-neoplastic cysts and 12 benign tumors. When categorized by the need for exploratory surgery (i.e., presence of a borderline tumor or malignant cancer), the sensitivity of this test was 85% and the specificity was 100%. These tests could inform the diagnosis of ovarian cysts and improve the clinical management of the large number of women with these lesions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods for protecting cells during cancer chemotherapy and radiotherapy
InactiveUS20050043224A1Protection from damagePrevent baldnessBiocideHeavy metal active ingredientsBone marrow transplantRadical radiotherapy
Compositions, pharmaceutical preparations and methods are disclosed for protecting non-neoplastic cells from damage caused by cancer chemotherapeutic agents or radiation therapy, during the course of cancer therapy or bone marrow transplant. These are based on the use of chemoprotective inducing agents that induce or increase production of cellular detoxification enzymes in target cell populations. The compositions and methods are useful to reduce or prevent hair loss, gastrointestinal distress and lesions of the skin and oral mucosa that commonly occur in patients undergoing cancer therapy. Also disclosed is a novel assay system for identifying new chemoprotective inducing agents.
Owner:WISCONSIN ALUMNI RES FOUND
Circulating tumour cell typing and identification kit
ActiveUS20170275696A1High detection sensitivityReducing false-negative resultMicrobiological testing/measurementFOXC2Wilms' tumor
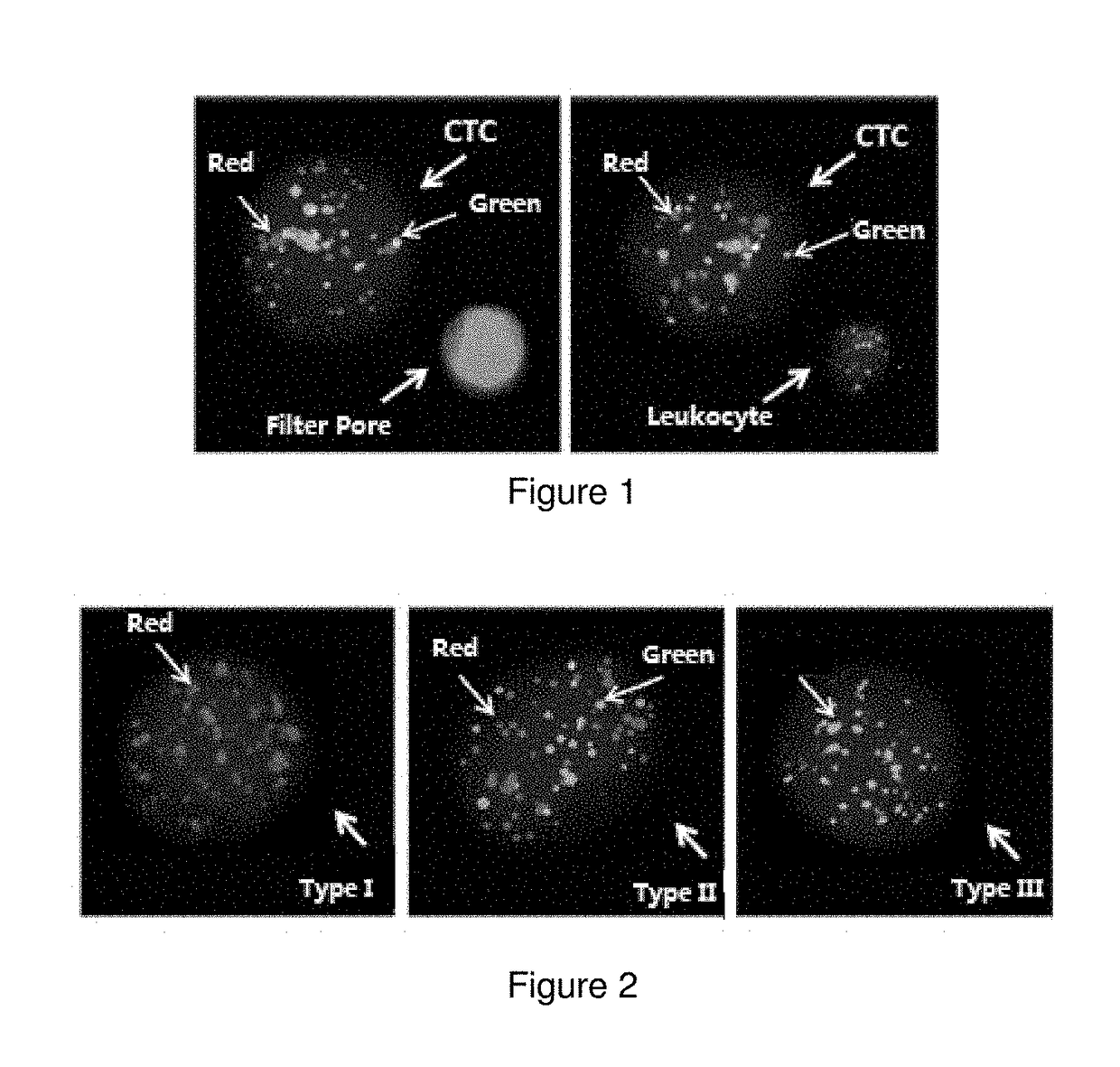
This disclosure relates to a circulating tumour cell typing and identification kit, comprising a capture probe, an amplification probe, and a labeled probe for each marker gene mRNA, wherein the marker gene mRNA comprises the following two types: at least two epithelial cell marker gene mRNAs selected from the group consisting of EPCAM, E-cadherin, CEA, KRT5, KRT7, KRT17, and KRT20 mRNAs; and, at least two mesenchymal cell marker gene mRNAs selected from the group consisting of VIMENTIN, N-cadherin, TWIST1, AKT2, ZEB2, ZEB1, FOXC1, FOXC2, SNAI1 and SNAI2 mRNAs. This disclosure prevents false-positive results caused by, for example, possible presence of a number of non-neoplastic epithelial cells in peripheral blood, introduction of normal epithelial cells during blood sampling, and the like. Accordingly, it may be assured that cells detected with epithelial cell marker genes and / or mesenchymal cell marker genes are indeed circulating tumour cells, further improving accuracy and reliability of the detection results.
Owner:SUREXAM BIO TECH
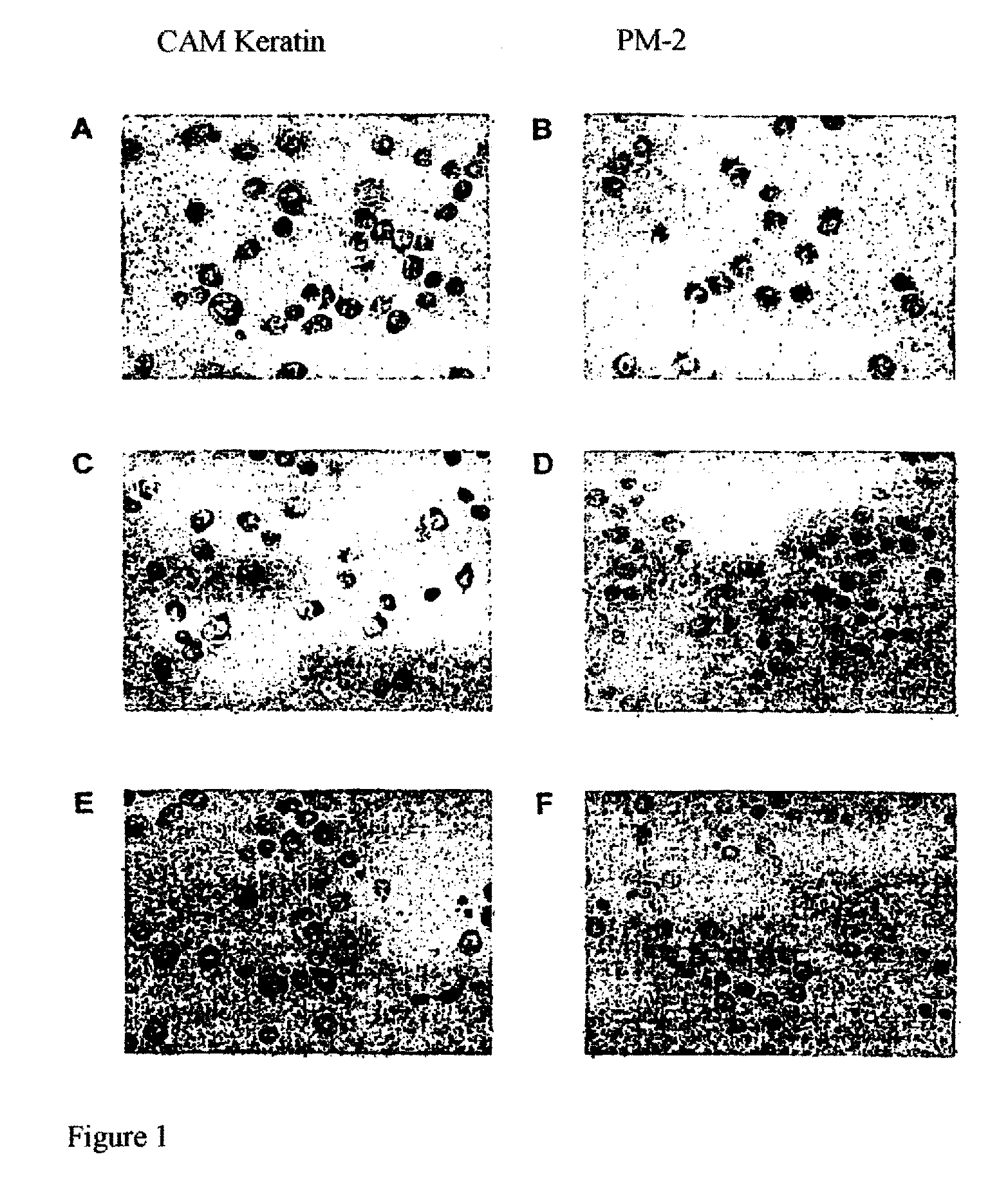
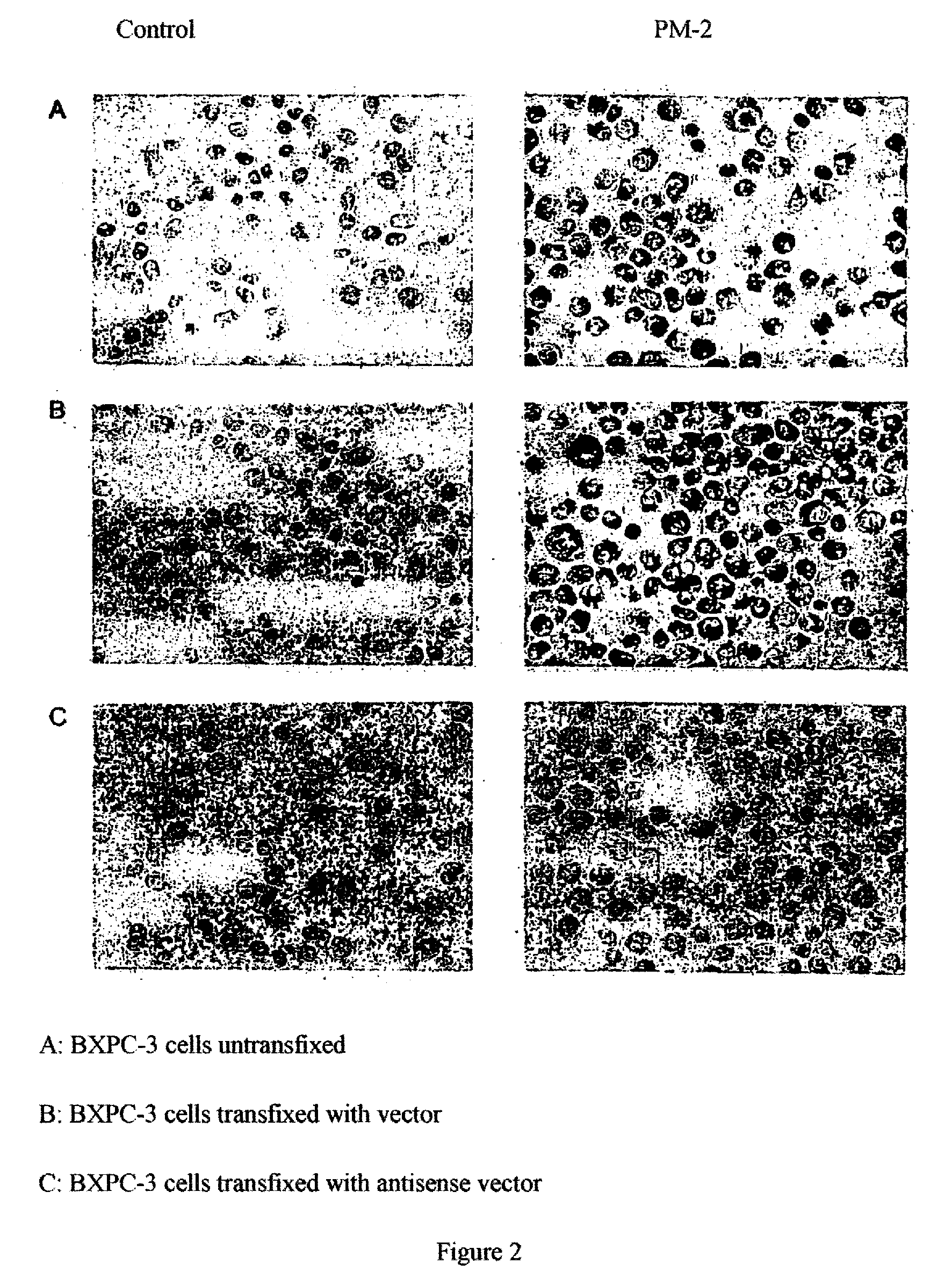
Antigen of the Pm-2 Antibody and Use Thereof
InactiveUS20080281083A1Cell receptors/surface-antigens/surface-determinantsDepsipeptidesAntigenMembrane bound protein
A polypeptide, which is expressed on the cell surface as a membrane-bound protein, is glycolyzed at one or more points (i.e., membrane glycoprotein) and has an amino acid sequence that corresponds partially or completely to that of the integrin binding protein p80 (accession #AJ131720) or REV1 (accession #AF206019.) The membrane glycoprotein, is expressed by neoplastic cells and not by non-neoplastic cells as an antigen, specifically binds the human monoclonal antibody PM-2 (DSM number: DSM ACC2600) and is, in addition, N-glycosidically and O-glucosidically linked. A related method is provided for the isolation or production of the antigen and for the use of the latter for producing a medicament for immunization. The isolated antigen can also be used to identify medicaments with an apoptotic cell-proliferation inhibiting action. The membrane glycoprotein can also be used as a tumor marker.
Owner:PATRYS
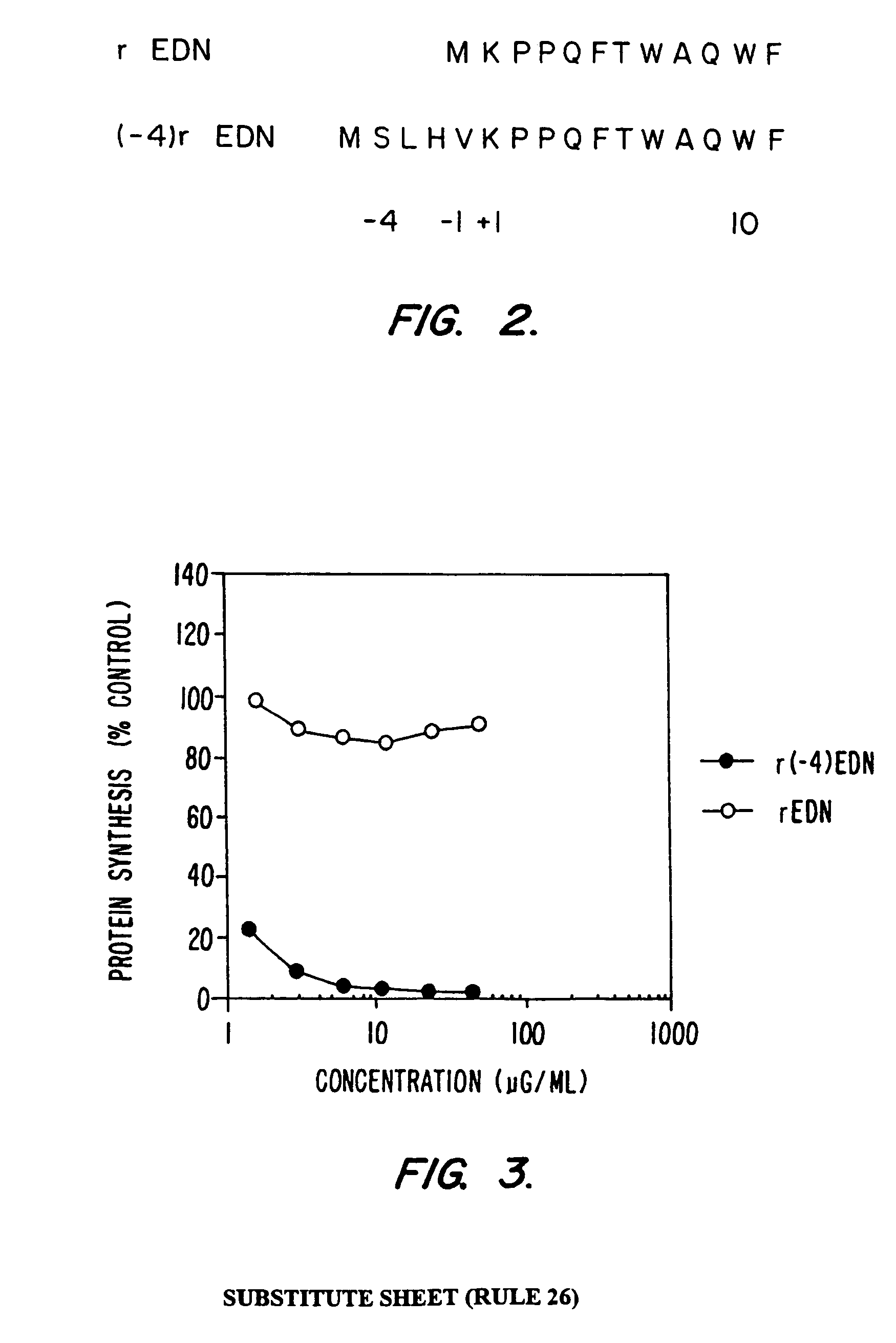
Selective toxicity of amino-terminal modified rnase a superfamily polypeptides
InactiveUS7029899B1Growth inhibitionAmelioration of Kaposi sarcomaSugar derivativesPeptide/protein ingredientsEndothelial NOSNeurexin
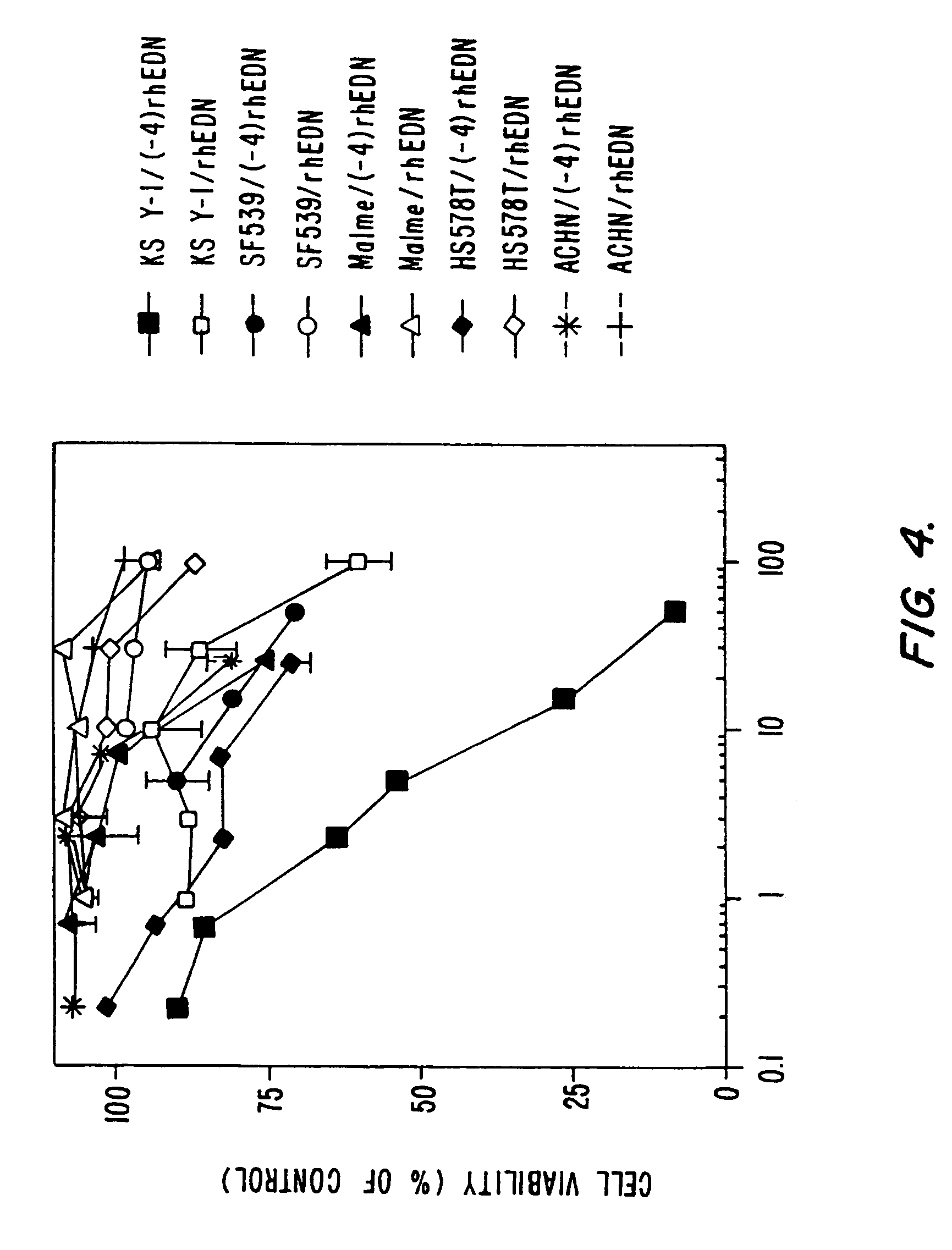
This invention provides RNase A superfamily polypeptides with modified amino terminal which can be used to selectively kill target Kaposi's sarcoma cells, neoplastic endothelial cells, and non-neoplastic endothelial cells. In certain embodiments of the invention, the amino terminal modification consists of an addition of 4 amino acid sequence consisting of the SLHV sequence at position −4 to −1 to the eosinophil derived neurotoxin protein. The amino terminal addition is capable of directing the claimed RNase A superfamily polypeptides to proliferating endothelial cells, such as Kaposi's sarcoma cells, and selectively killing these cells.
Owner:HEALTH & HUMAN SERVICES DEPT OF GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF
Epha2 and hyperproliferative cell disorders
InactiveUS20090162933A1Promote migrationIncrease secretionSenses disorderDigestive systemCell phenotypeDisease
The present invention relates to methods and compositions designed for the treatment, management, or prevention of a non-neoplastic hyperproliferative cell or excessive cell accumulation disorders, particularly those involving hyperproliferation of epithelial or endothelial cells. In one embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and increase EphA2 cytoplasmic tail phosphorylation and / or increase EphA2 autophosphorylation, in cells which EphA2 has been agonized. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and reduce EphA2 activity (other than autophosphorylation). In another embodiment, the methods of the invention comprise administration of an effective amount of one or more EphA2 agonistic agents that bind to EphA2 and decrease a pathology-causing cell phenotype (e.g., a pathology-causing epithelial cell phenotype or a pathology-causing endothelial cell phenotype). In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more EphA2 agonistic agents that are EphA2 antibodies that bind to EphA2 with a very low Koff rate. In preferred embodiments, agents of the invention are monoclonal antibodies. The invention also provides pharmaceutical compositions comprising one or more EphA2 agonistic agents of the invention either alone or in combination with one or more other agents useful in therapy for non-neoplastic hyperproliferative cell or excessive cell accumulation disorders.
Owner:KIENER PETER A +3
Inherited Mitochondrial Dna Mutations in Cancer
InactiveUS20080280294A1Increase chanceSugar derivativesMicrobiological testing/measurementProstate cancerFhit gene
A method is provided for identifying a subject likely to have, or at risk of developing a disease condition correlated with increased reactive oxygen species (ROS), including cancer, by identifying in the subject a missense mutation in a nucleic acid of Complex III, IV and / or V of the OXPHOS system. This invention also provides a method of identifying a likelihood of having a heritable predisposition to cancer by detecting a homoplasmic missense mutation in non-tumor tissue of an OXPHOS system gene. This invention also provides a method for detecting likelihood of having cancer, predisposition to cancer, and likelihood of passing a predisposition to cancer to progeny involving identifying in non-tumor tissue of the subject a missense mutation in a complex III, IV and / or V gene of the mitochondrial OXPHOS system. The mutation may be a nuclear or mitochondrial mutation. The invention has been exemplified with respect to prostate cancer. When the mutation is homoplasmic in non-tumor tissue this is an indication it is an inherited and inheritable trait, and that the subject is likely to pass on the mutation to her progeny in the case of mutations in mitochondrial DNA or his or her progeny in the case of mutations in nuclear DNA. Both homoplasmic and heteroplasmic mutations in non-tumor tissue can indicate the presence of cancer.
Owner:EMORY UNIVERSITY
Use of vinca alkaloids and salts thereof
InactiveUS20090239301A1Increasing insulin-producingOrganic active ingredientsSenses disorderAdditive ingredientMedicine
An agent containing a vinca alkaloid or its pharmacologically acceptable salt as an active ingredient can induce insulin production and / or secretion of non-neoplastic cells derived from the pancreas.
Owner:KEIO UNIV
Treatment of Hematologic Disorders
InactiveUS20090028870A1Reduce GVHDRemarkable effectBiocideOrganic active ingredientsGraft versus leukaemiaHematological Diseases
Owner:THE GENERAL HOSPITAL CORP
Combination therapy and methods for treatment and prevention of hyperproliferative diseases
InactiveUS20150094277A1Prevention and treatmentSafe and well-toleratedBiocideInorganic boron active ingredientsDiseaseSide effect
The present invention provides therapy and methods for the treatment and prevention of diseases of cell proliferation such as cancer, benign tumors, and viral diseases such as HIV-AIDS, hepatitis B, hepatitis C and cirrhosis. The methods of this invention consist of the administration to a patient of a combination of effective amounts of agents capable of eradicating the neoplastic cells, while sparing the non-neoplastic cells from cytotoxic side-effects. The agents co-administered in therapeutically effective amounts are: chemotherapeutic agents, apoptotic agents, anti-angiogenic agents, cell differentiation agents, immunomodulating agents, antioxidants, vitamins, microelements, enzymes and natural extracts.
Owner:CIUSTEA MIHAI
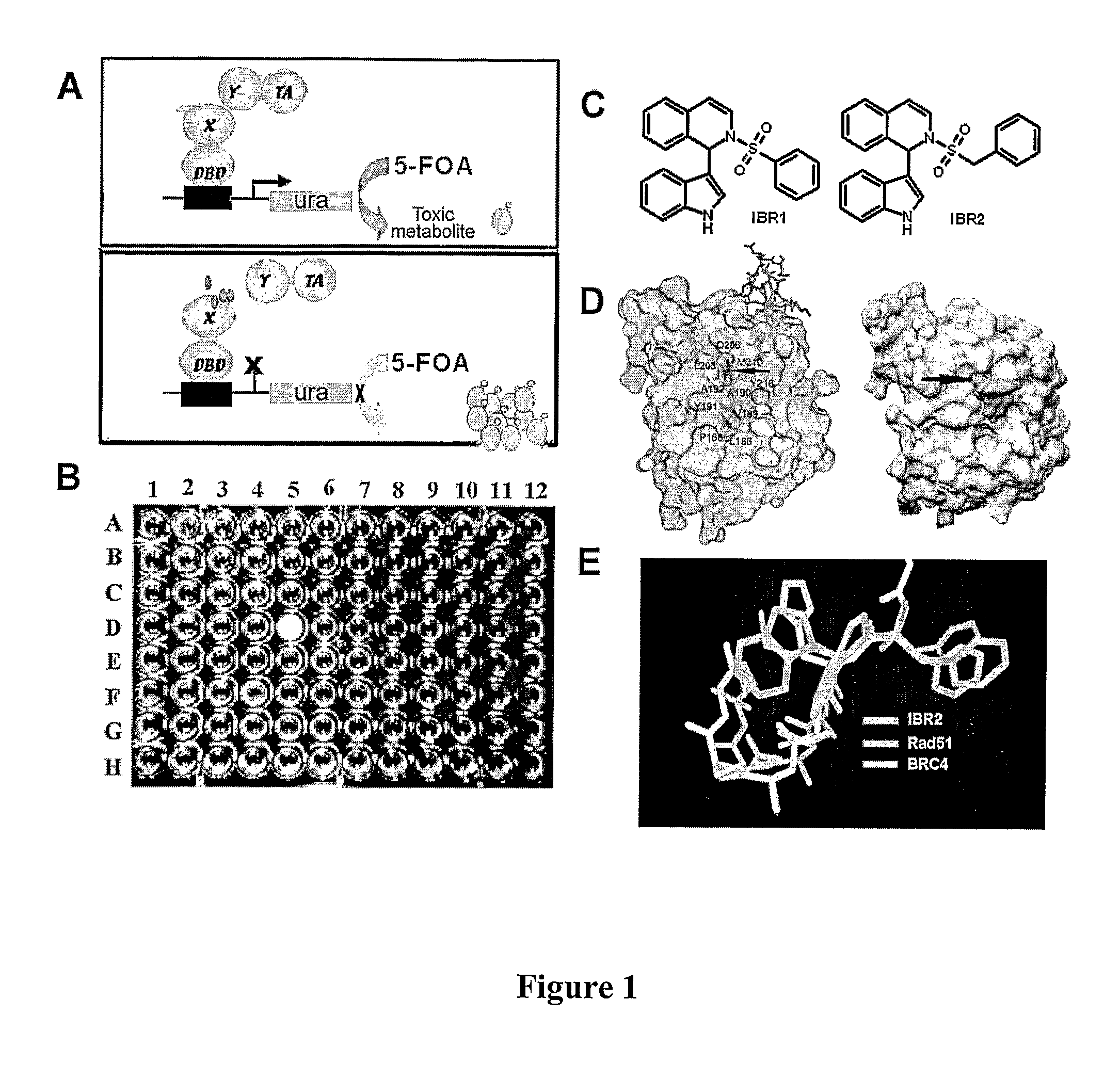
Compositions and methods for disruption of BRCA2-Rad51 interaction
Contemplated compounds disrupt interaction between BRCA2 and RAD51, likely by binding to RAD51. Based on the crucial role of the BRCA2-RAD51 complex formation in DNA repair and the role of RAD51 in the control of entry into S-phase from G1, numerous compositions and methods are presented. Among other advantageous uses, contemplated compounds may be employed as protective agents for non-neoplastic cells in chemotherapy before exposure of the cells to a chemotherapeutic drug, and / or as DNA-damage sensitizer for neoplastic cells.
Owner:RGT UNIV OF CALIFORNIA
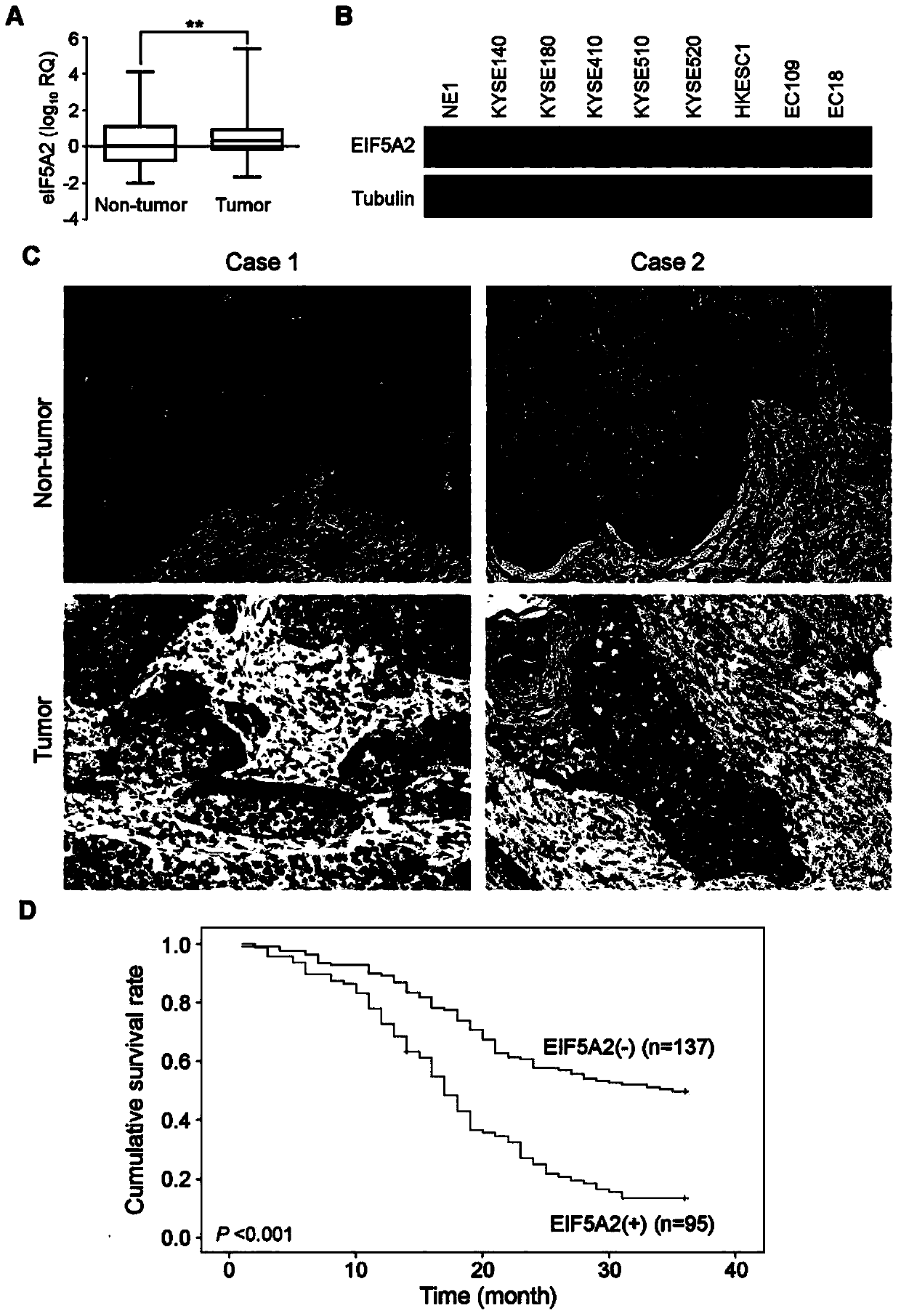
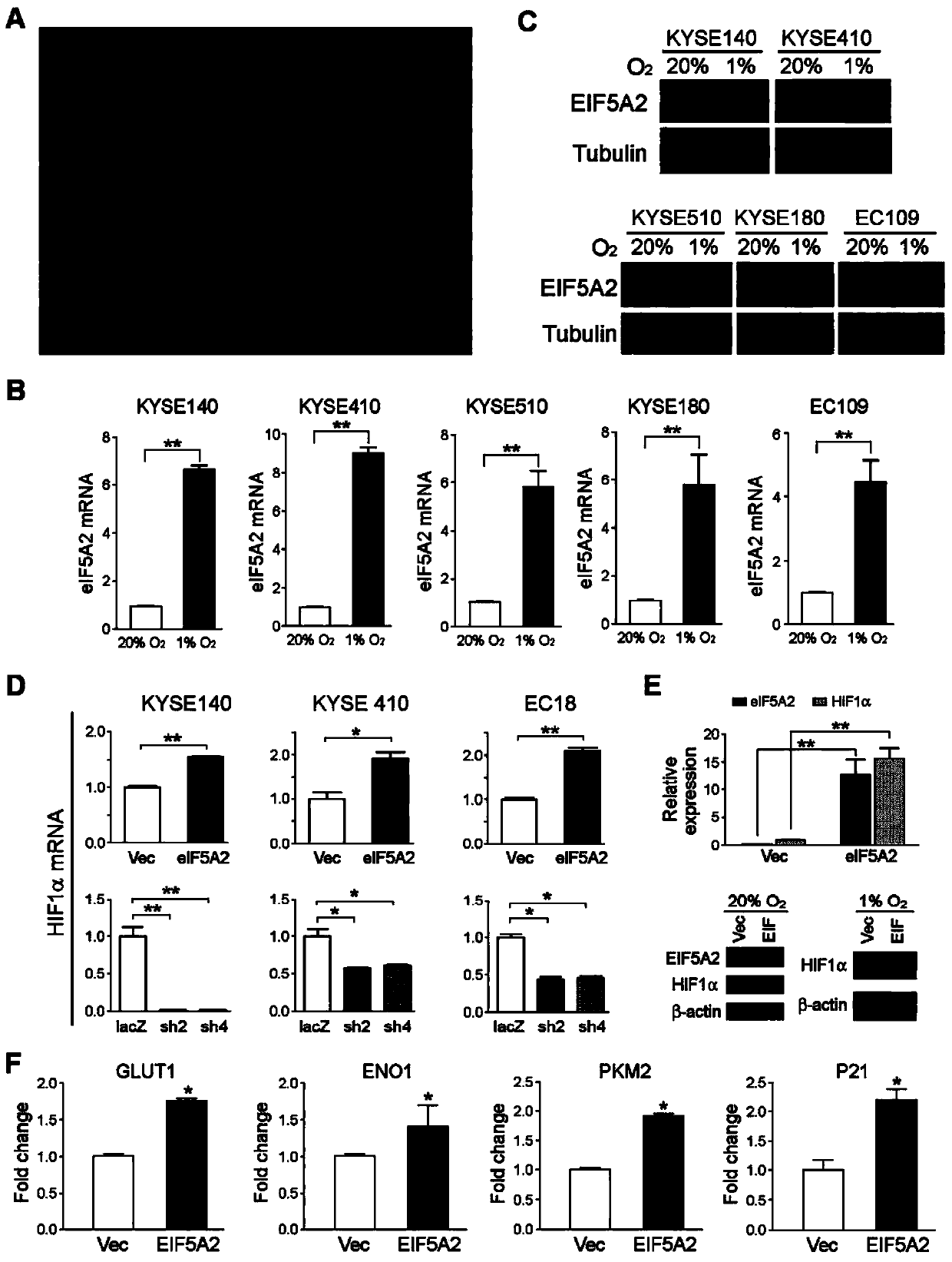
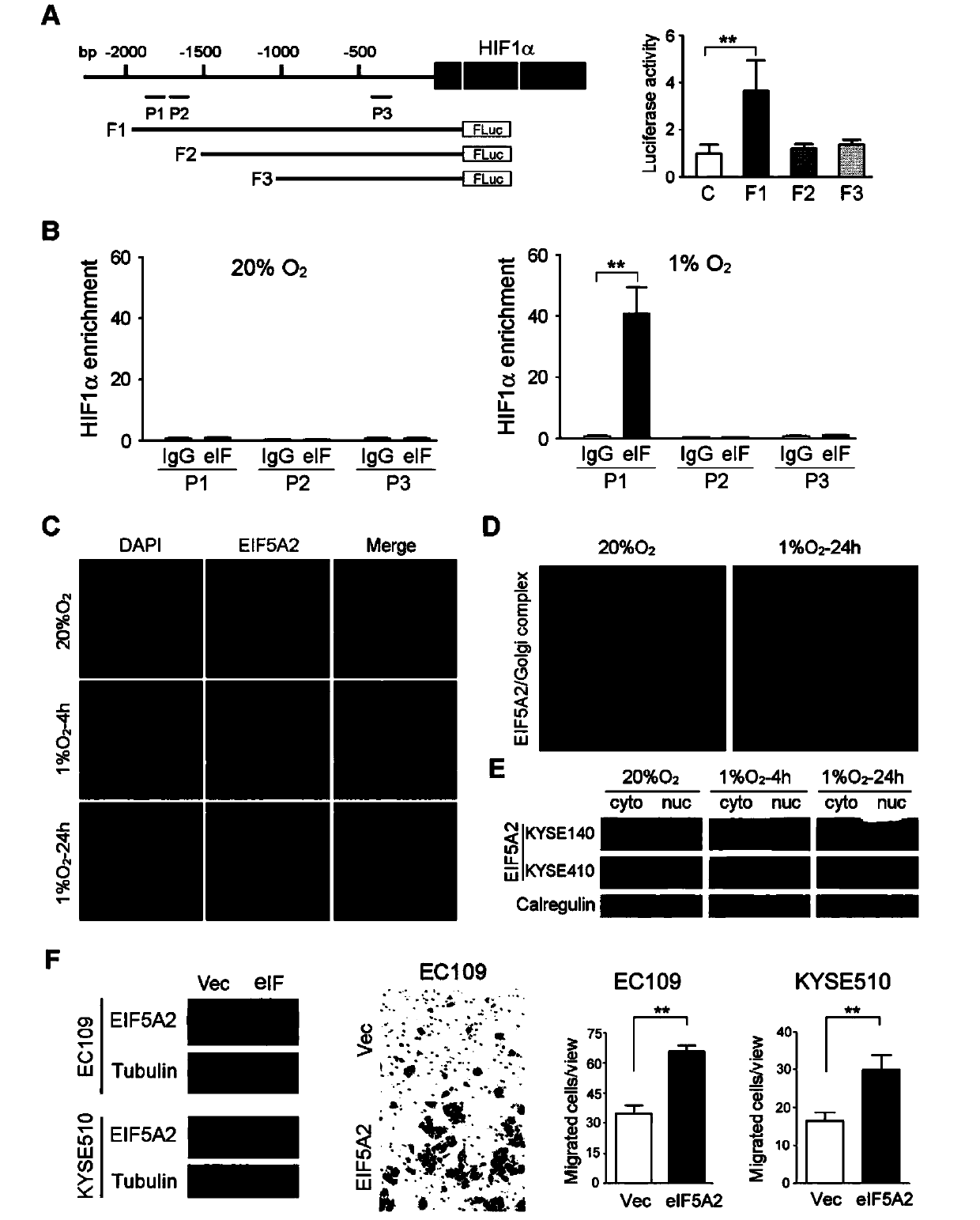
Application of EIF5A2 to preparation of esophageal squamous cell carcinoma prognosis reagent
ActiveCN103468799APromote formationPromote invasionMicrobiological testing/measurementMaterial analysisOxygen deprivationNode metastasis
The invention discloses application of EIF5A2 to preparation of an esophageal squamous cell carcinoma prognosis reagent. Real-time quantitative PCR (Polymerase Chain Reaction) detection data shows that expression of the EIF5A2 in esophageal squamous cell carcinoma tissues is obviously higher than that in corresponding non-tumor tissues. An immunohistochemical data analysis result shows that expression of the EIF5A2 is positively correlated to lymphatic metastasis, the tumor invasion depth and neoplasm staging. Kaplan-Meier analysis shows that a patient overexpressed by the EIF5A2 has poor overall survival (p is less than 0.001). Multivariate analysis also proves that the EIF5A2 is an independent prognostic factor. The EIF5A2 possibly takes important effects in invasion and metastasis of esophageal squamous cell carcinoma. Further experimental data proves that overexpression of the EIF5A2 can be caused by gene amplification and oxygen deprivation; the EIF5A2 can be combined into a promoter region of HIF1a to up-regulate expression of the HIF1a; and the EIF5A2 can induce epithelial-mesenchymal transition conversion and promote angiopoiesis of the esophageal squamous cell carcinoma so as to promote invasion and metastasis of the esophageal squamous cell carcinoma. Knockout of the EIF5A2 can obviously inhibit tumor metastasis and the silent EIF5A2 is expected to be a potential target of esophageal squamous cell carcinoma treatment.
Owner:SUN YAT SEN UNIV CANCER CENT
Topographic genotyping for determining the diagnosis, malignant potential, and biologic behavior of pancreatic cysts and related conditions
InactiveUS20140296103A1Easy to analyzeEasy diagnosisHealth-index calculationMicrobiological testing/measurementMolecular analysisMicrosatellite
The application relates to a method of a predicting the presence of invasive pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions comprising detailed molecular analysis incorporating DNA quality and quantity, K-ras mutational analysis and a broad spectrum of tumor suppressor gene linked microsatellite LOH. Methods of diagnosing, determining prognosis of and determining a course of treatment for pancreatic cancer or high grade dysplasia, pre-cancerous pancreatic states and non-neoplastic conditions are also provided.
Owner:REDPATH INTEGRATED PATHOLOGY INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com