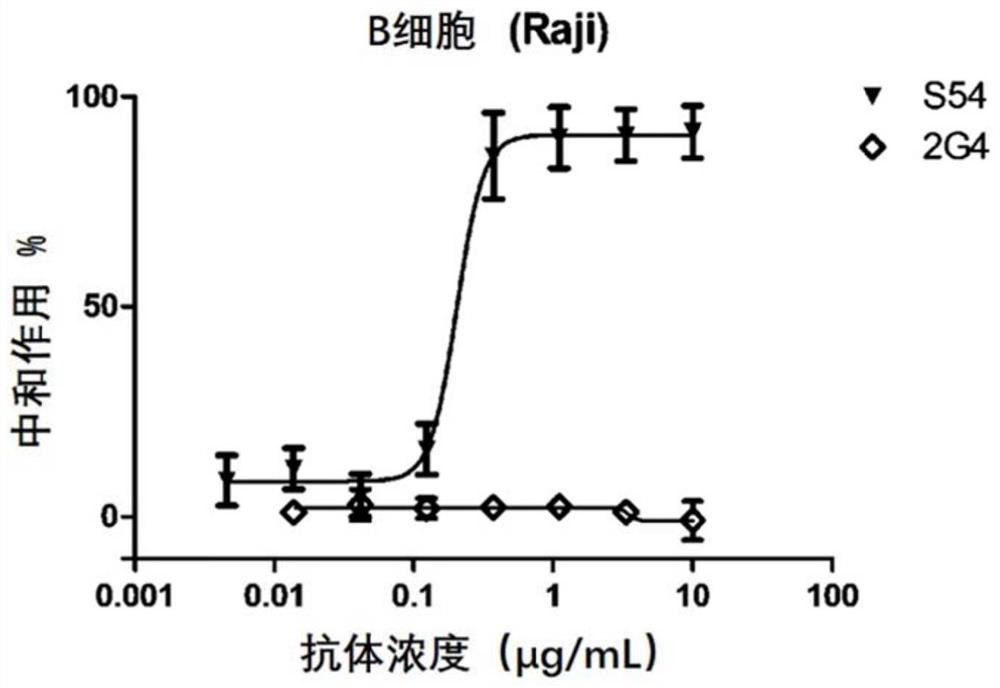
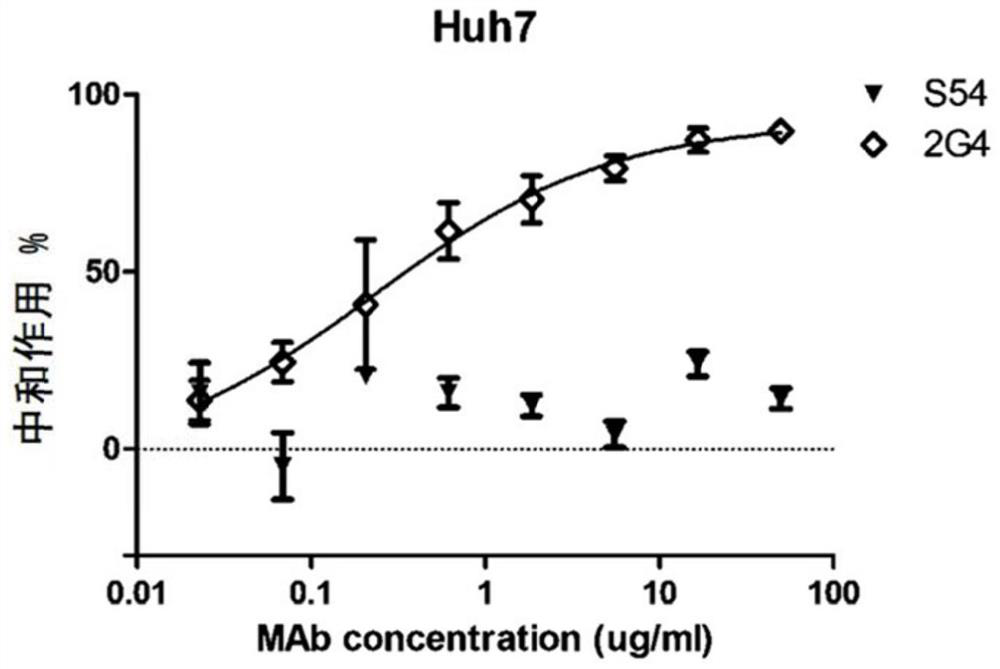
A kind of monoclonal antibody and application thereof for neutralizing Epstein-Barr virus
An Epstein-Barr virus, antibody technology, applied in the direction of antibodies, applications, antiviral agents, etc., can solve the problems of human monoclonal antibodies and other issues, and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of EBV gp350 recombinant protein
[0040] The gp350 protein plays an important role in the process of EBV B cells. Therefore, the inventor's research team chose gp350 as the bait protein to screen the scFv library displayed on the surface of yeast, in order to obtain neutralizing antibodies that can block EBV infection.
[0041] Then, the genome of EBV-M81 strain was selected as a template to amplify the sequence of gp350.
[0042] DNA sequence of gp350:
[0043] ATGCCCATGGGGTCTCTGCAACCGCTGGCCACCTTGTACCTGCTGGGGATGCTGGTCGCTTCCTGCCTCGGAATGGAGGCAGCCTTGCTTGTGTGTCAGTACACCATCCAGAGCCTGATCCATCTCACGGGTGAAGATCCTGGTTTTTTCAATGTTGAGATTCCGGAATTCCCATTTTACCCCACATGCAATGTTTGTACGGCAGATGTCAATGTAACTATCAATTTCGATGTCGGGGGCAAAAAGCATCAACTTGATCTTGACTTTGGCCAGCTGACACCCCATACGAAGGCTGTCTACCAACCTCGAGGTGCATTTGGTGGCTCAGAAAATGCCACCAATCTCTTTCTACTGGAGCTCCTTGGTGCAGGAGAATTGGCTCTAACTATGCGGTCTAAGAAGCTTCCAATTAACGTCACCACCGGAGAGGAGCAACAAGTAAGCCTGGAATCTGTAGATGTCTACTTTCAAGATGTGTTTGGAACCATGTGGTGC...
Embodiment 2
[0079] Example 2 Screening of yeast library
[0080] Using yeast library screening technology, the positive clones of yeast specifically binding to gp350 protein were obtained.
[0081] (1) Materials and equipment
[0082] Yeast surface display human non-immune scFv library is the antibody heavy chain and light chain variable regions respectively amplified from 58 normal human spleen and lymph node lymphocytes, with 3 G in the middle 4 The S linker constructs two genes together on the yeast display vector, that is, successfully constructs the yeast surface display scFv library, and the library capacity of the library is 10 9 (The yeast surface display scFv library used in this experiment comes from Michael J. laboratory); Omega Yeast Plasmid Small Extraction Kit; Amp+ plate; LB medium; streptavidin-microbeads (miltenyi biotec); anti-biotin-microbeads (miltenyi biotec) ; streptavidin-APC (ebioscience); anti-biotin-PE (ebioscience); YPD medium; SDCAA medium; SDCAA plate; SGCAA...
Embodiment 3
[0086] Example 3 Sequencing and Expression Purification of Monoclonal Antibodies
[0087] (1) The human-derived scFv yeast positive clones combined with gp350 were screened in Example 2, the plasmid in the yeast was extracted, and the antibody gene was obtained by sequencing.
[0088] (2) Expression and purification of monoclonal antibodies. The screened antibody heavy chain variable region upstream and CMV fragment, downstream and human IgG1 constant region and poly A fragment were subjected to overlapping PCR to obtain a fragment that could express the complete heavy chain; the screened antibody light chain variable region The upstream and CMV fragments, the downstream and the light chain κ / λ constant region, and the poly A fragment were subjected to overlapping PCR to obtain fragments that could express the complete light chain. These two fragments were then constructed into the PMD18T (Takara) vector. The PMD18T plasmid containing the full-length sequence of the antibody...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com