Patents
Literature
45 results about "Ebola virus infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
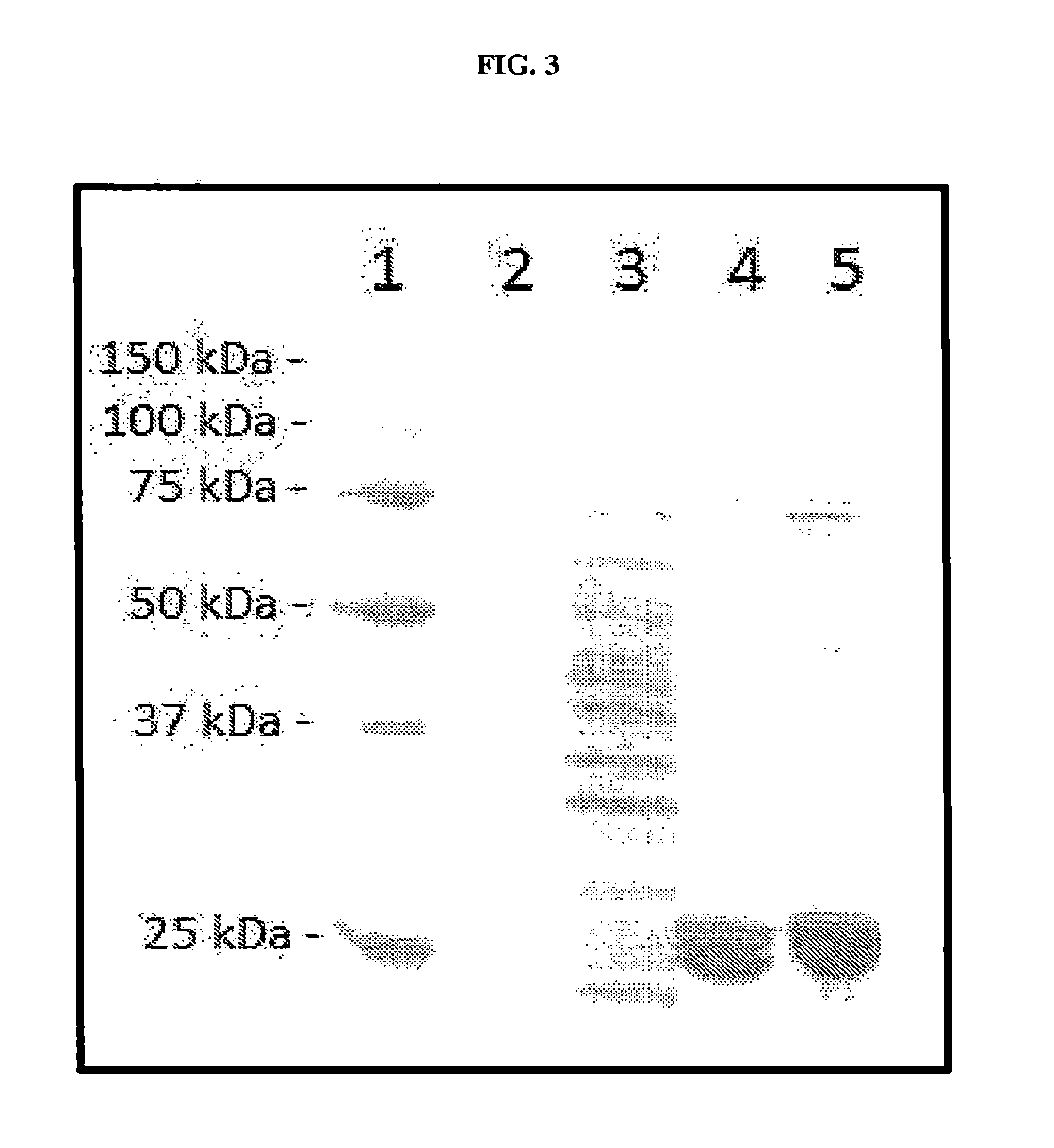
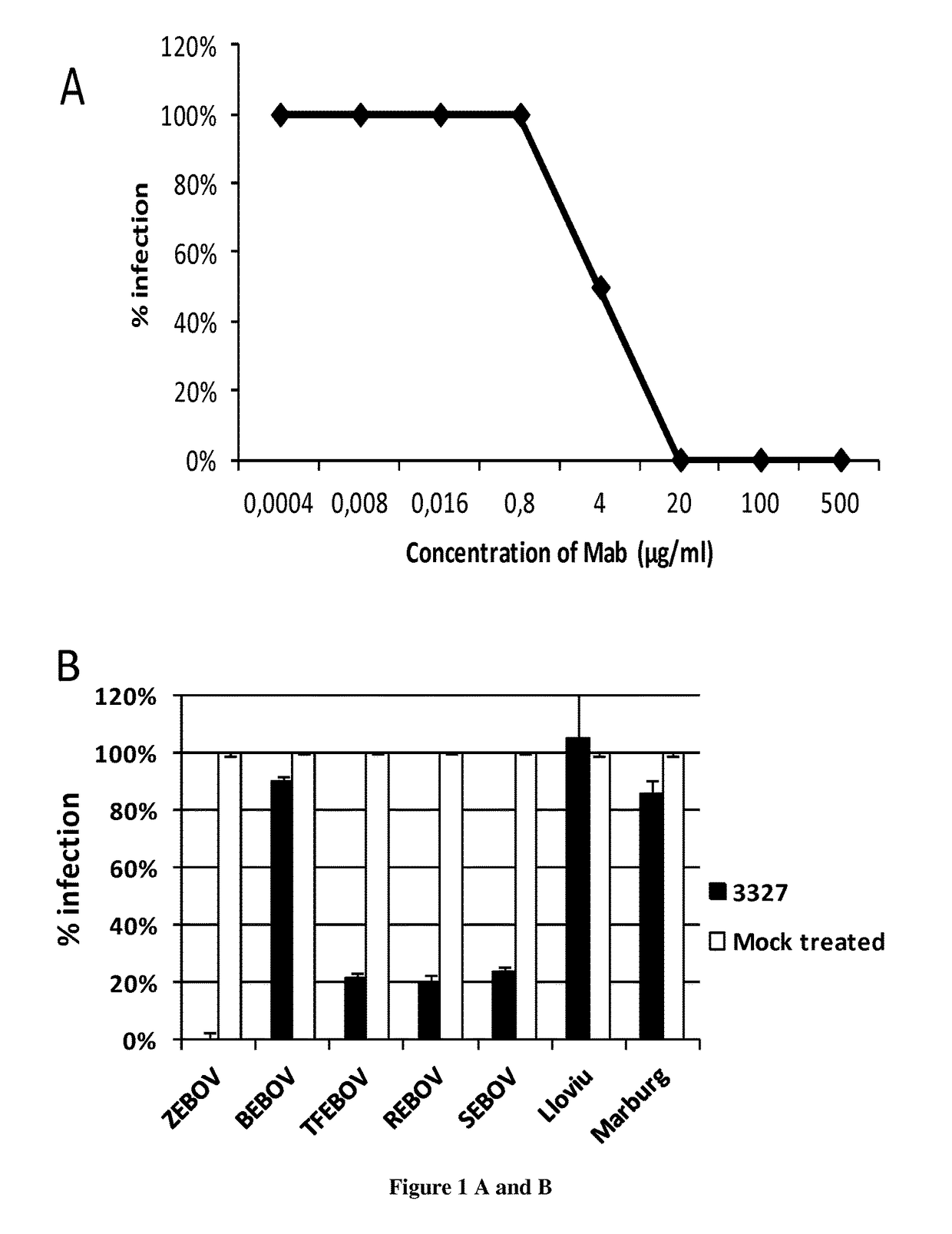
Human Antibodies to Ebola Virus Glycoprotein
ActiveUS20160215040A1Inhibiting and neutralizing activityAvoid enteringImmunoglobulins against virusesAntiviralsViral glycoproteinAntigen Binding Fragment
The present invention provides monoclonal antibodies, or antigen-binding fragments thereof, that bind to Ebola virus glycoproteins, pharmaceutical compositions comprising the antibodies and methods of use. The antibodies of the invention are useful for inhibiting or neutralizing Ebola virus activity, thus providing a means of treating or preventing Ebola virus infection in humans. In some embodiments, the invention provides for use of one or more antibodies that bind to the Ebola virus for preventing viral attachment and / or entry into host cells. The antibodies of the invention may be used prophylactically or therapeutically and may be used alone or in combination with one or more other anti-viral agents or vaccines.
Owner:REGENERON PHARM INC
Monoclonal antibodies and complementarity-determining regions binding to Ebola glycoprotein
InactiveUS7335356B2Animal cellsMicrobiological testing/measurementEpitopeComplementarity determining region
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Methods for prevention and treatment of infections with supraphysiological doses of mannan-binding lectin (MBL) and ficolin-mbl fusion proteins
InactiveUS20100331240A1Polypeptide with localisation/targeting motifAntibacterial agentsBacteroidesInfection risk
The present invention provides methods of treatment and / or prevention of infections, for example, viral and bacterial infections, in individuals, wherein the method comprises administering a supraphysiological amount of mannose-binding lectin (MLB) and / or ficolin-MBL fusion protein to an individual afflicted with an infection or at risk of an infection, such as a bacterial or a viral infection. For example, methods for treatment and / or prevention of Ebola virus infection are provided.
Owner:THE GENERAL HOSPITAL CORP
Human antibodies to ebola virus glycoprotein
ActiveUS9771414B2Inhibiting and neutralizing activityAvoid enteringPeptide/protein ingredientsVirus peptidesAntigenAntigen Binding Fragment
The present invention provides monoclonal antibodies, or antigen-binding fragments thereof, that bind to Ebola virus glycoproteins, pharmaceutical compositions comprising the antibodies and methods of use. The antibodies of the invention are useful for inhibiting or neutralizing Ebola virus activity, thus providing a means of treating or preventing Ebola virus infection in humans. In some embodiments, the invention provides for use of one or more antibodies that bind to the Ebola virus for preventing viral attachment and / or entry into host cells. The antibodies of the invention may be used prophylactically or therapeutically and may be used alone or in combination with one or more other anti-viral agents or vaccines.
Owner:REGENERON PHARM INC
Monoclonal antibody of human anti-Ebola virus envelope glycoprotein, and application thereof
ActiveCN107033242AAct as an infectionHigh activityImmunoglobulins against virusesAntiviralsNPC1Glycan
The invention discloses a monoclonal antibody of a human anti-Ebola virus envelope glycoprotein GP. The antibody has a unique CDR region and has a unique action site. Computer simulation analysis and biological experiments confirm that the antibody acts on the NPC1 receptor binding region of the GP, and also acts on the glycan cap region of the GP, so the antibody plays a role in the anti-Ebola virus infection through the unique neutralizing mechanism formed by the combined action of the antibody on the two structural domains of the GP. The antibody has high antigen binding activity and neutralizing activity, can protect 50% of virus attacked cells just at dose of 0.3 [mu]g / ml, and has a protection rate reaching 100% at an antibody concentration of 100 [mu]g / ml, so the antibody provided by the invention has a wide application prospect in the preparation of Ebola virus disease treatment medicines.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
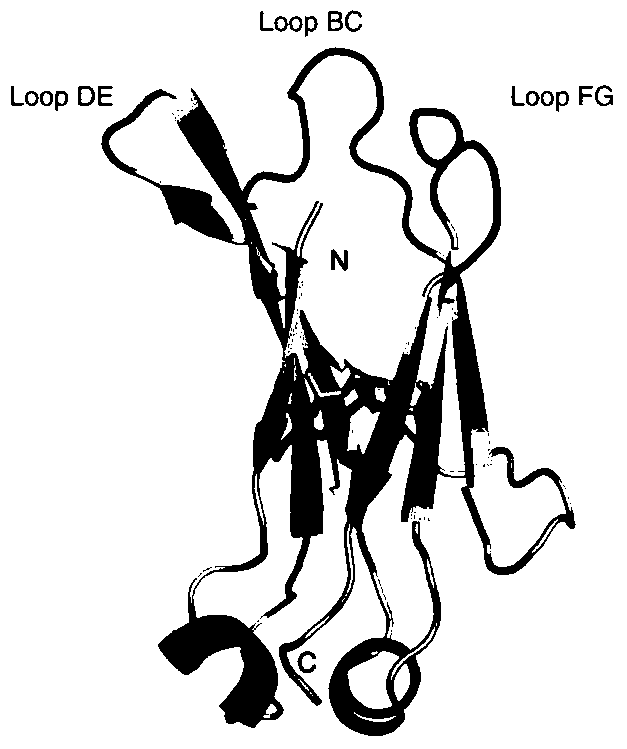
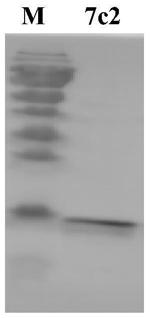
Nanometer antibody for neutralizing Ebola viruses
ActiveCN106188286AGood tissue permeabilityReduce manufacturing costMaterial nanotechnologyBiological material analysisNucleotideHalf-life
The invention provides a nanometer antibody ebo7c2 for neutralizing Ebola viruses and application of the nanometer antibody to preparing medicines for preventing or treating Ebola virus infection. Amino acid sequences of the nanometer antibody are shown as SEQ ID NO:1, and nucleotide sequences for genes for encoding the nanometer antibody ebo7c2 are shown as SEQ ID NO:2. The nanometer antibody and the application have the advantages that CH2 structural domains m01s of human antibodies IgG1 are used as skeletons of the nanometer antibody, and amino acid of the nanometer antibody is different from amino acid sequences of the CH2 structural domains m01s in three loop zones; the nanometer antibody ebo7c2 can be bound with envelope proteins of the Ebola viruses; the nanometer antibody ebo7c2 has small molecular weights and is excellent in tissue permeability and ability of being bound with antigen epitopes with steric hindrance effects; the nanometer antibody is provided with the m01s skeletons and can be bound with FcRn, and the plasma half-life of the nanometer antibody can possibly reach 10 hours; the nanometer antibody ebo7c2 can be expressed in prokaryotic expression systems and is low in production cost and short in cycle.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Monoclonal antibodies and complementarity-determining regions binding to ebola glycoprotein
InactiveUS20070298042A1Animal cellsMicrobiological testing/measurementEpitopeComplementarity determining region
In this application are described Ebola GP monoclonal antibodies, epitopes recognized by these monoclonal antibodies, and the sequences of the variable regions of some of these antibodies. Also provided are mixtures of antibodies of the present invention, as well as methods of using individual antibodies or mixtures thereof for the detection, prevention, and / or therapeutical treatment of Ebola virus infections in vitro and in vivo.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Application of berbamine dihydrochloride in preparing Ebola virus inhibitor
ActiveCN109125323AAchieve the effect of infectionEnhanced inhibitory effectOrganic active ingredientsAntiviralsProtein targetVirtual screening
The invention discloses an application of berbamine dihydrochloride in preparing Ebola virus inhibitor. According to the invention, the envelope glycoprotein (EBOV-GPcl) of the Ebola virus in an activated state is taken as a target point, and an antiviral active compound which is capable of binding with the EBOV-GPcl is obtained through structure-based virtual screening, and the compound is the berbamine dihydrochloride. The berbamine dihydrochloride can specifically inhibit the entry of the Ebola recombinant virus by combining with the target protein EBOV-GPcl so as to achieve the effect of resisting Ebola virus infection. The semi-maximal effect concentration (EC50) of the berbamine dihydrochloride against the EBOV is 0.49 micrometer, indicating that the berbamine dihydrochloride has a strong inhibitory effect on the EBOV.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Biological preparation and application of same in preparation of drugs used for preventing and controlling Ebola virus
ActiveCN105031623AImprove stabilityStop spreading infectionPeptide/protein ingredientsAntiviralsProtein solutionGenotype
The invention discloses a biological preparation used for preventing and controlling multiple genotypes of Ebola virus infection. A preparation method for the biological preparation comprises the following steps: preparing a mother liquor with a concentration of 1 M from acid anhydride and dimethyl sulfoxide; preparing a disodium hydrogen phosphate solution with a concentration of 0.1 M and applying the disodium hydrogen phosphate solution in preparation of a protein solution with a protein concentration of 20 mg / ml; adding the acid anhydride solution into the to-be-modified protein solution, wherein the concentration of added acid anhydride is allowed to be 12 mM, carrying out full and uniform mixing, adjusting the pH value of the protein solution to 9.0 and carrying out incubation at 25 DEG C for 20 min; repeating mixing of the acid anhydride and the protein solution until eventually, the final concentration of the acid anhydride in the protein solution is 60 mM and the pH value of the solution is 9.0, and carrying out incubation at 25 DEG C for 2 h to accomplish acid anhydride reaction; and filling a dialysis bag with the acid anhydridated protein solution, putting the dialysis bag in a phosphatic buffer with a pH value of 7.4 for dialysis for 48 h, filtering the acid anhydridated protein solution with a microfiltration membrane with a size of 0.45 [mu]m and carrying out preservation at 4 DEG C.
Owner:FUDAN UNIV +1
Active molecule capable of suppressing gene replication of Ebola virus and usage method thereof
InactiveCN105063044AGood curative effectCompounds screening/testingPowder deliveryLipid formationRed blood cell
The invention relates to an active molecule capable of suppressing gene replication of Ebola virus. The active molecule is compsoed of nanogranule formulation of HKP or SLiC or RPH vector and siRNA cocktail for preventing and treating Ebola virus infection. The active molecule specificly relates to the siRNA molecule cocktain of targeted Ebola virus, cocktail siRNA composition of targeted Ebola virus gene conservative region, nanoparticle of siRNA cocktail and Histidine-lysine copolymer (HKP), or / and spermine-lipid-cholesterol (SLiC)nanoparticle formulation composition; composition of siRNA cocktail and HKP or SLiC nanoparticle formulation. The formulation is modified by Arg-Gly-Asp(RGD) polypeptide ligands which is specific to a endothelial cell surface acceptor or erythrocyte, and is called RGD-PEG-HKP(RPH) vector system. The invention also relates to a method for testing new siRNA formualtion in cell cultrue, a method for testing new siRNA formulation in guinea pig model and monkey model and a method for testing nanoparticle-containing siRNA formualtion in injection liquid for human.
Owner:SIRNAOMICS BIOPHARMACEUTICALS (SUZHOU) CO LTD
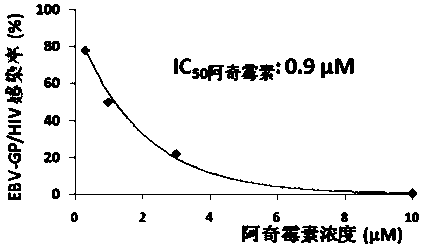
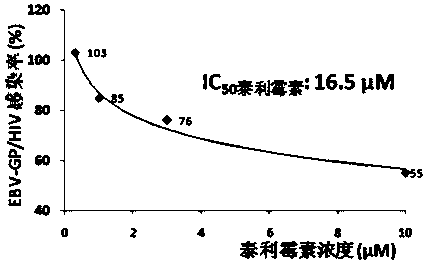
Applications of azithromycin and telithromycin in anti-Ebola virus infection
ActiveCN105362284AClear securityDrug metabolism properties are clearOrganic active ingredientsAntiviralsAnti virusAzithromycin
The present invention discloses applications of azithromycin and telithromycin in anti-Ebola virus infection, wherein the applications comprise applications of azithromycin or / and telithromycin in prevention or treatment of Ebola virus infection or combined applications of azithromycin or / and telithromycin and other anti-virus drugs.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
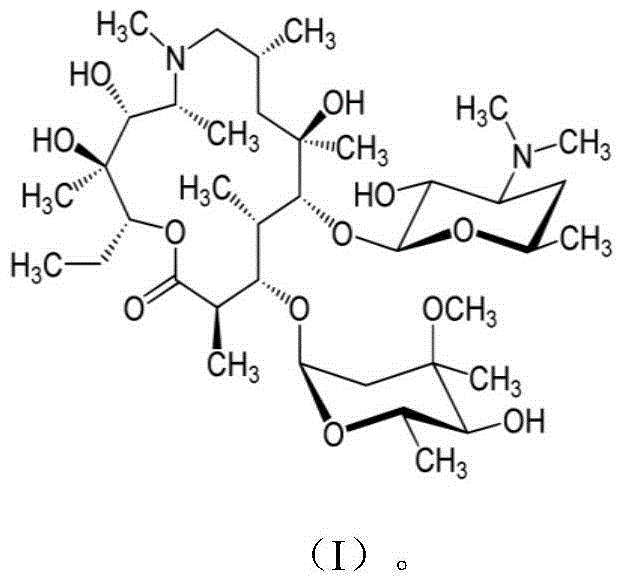
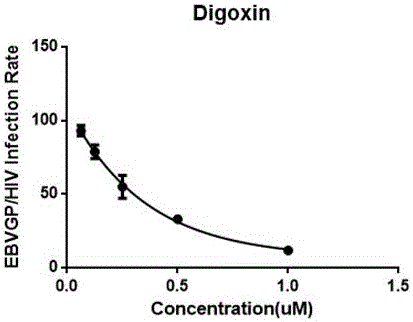
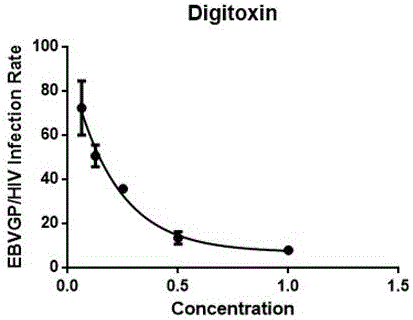
Applications of cardiac glycoside compound in resisting Ebola virus infection
InactiveCN106562982AClear securityDrug metabolism properties are clearOrganic active ingredientsAntiviralsPhases of clinical researchCardiac glycoside
The invention discloses applications of cardiac glycoside medicines in resisting Ebola virus infection. The infection stages comprise invasion, transcription and replication; the cardiac glycoside medicines comprise but are not limited to digoxin, digitoxin, lanatoside C and ouabain G. The four representative cardiac medicines can effectively prevent the Ebola virus from infecting host cells, thus a theoretical basis is provided for developing the antiviral compound medicines taking the cardiac glycoside as the main ingredient, and meanwhile, a scientific and technical support is provided for preventing and controlling the viral emerging infectious diseases.
Owner:SUZHOU INST OF SYST MEDICINE
Human antibodies to ebola virus glycoprotein
ActiveUS20170355751A1Inhibiting and neutralizing activityAvoid enteringImmunoglobulins against virusesAntiviralsViral glycoproteinAntigen Binding Fragment
The present invention provides monoclonal antibodies, or antigen-binding fragments thereof, that bind to Ebola virus glycoproteins, pharmaceutical compositions comprising the antibodies and methods of use. The antibodies of the invention are useful for inhibiting or neutralizing Ebola virus activity, thus providing a means of treating or preventing Ebola virus infection in humans. In some embodiments, the invention provides for use of one or more antibodies that bind to the Ebola virus for preventing viral attachment and / or entry into host cells. The antibodies of the invention may be used prophylactically or therapeutically and may be used alone or in combination with one or more other anti-viral agents or vaccines.
Owner:REGENERON PHARM INC
Ebola virus typing fluorescence PCR detection kit
InactiveCN104711370AEasy to operateSimple methodMicrobiological testing/measurementRNA extractionMagnetic bead
The invention provides an ebola virus typing fluorescence PCR detection kit, the kit comprises a PCR reaction solution for detecting a primer probe sequence of Zaire ebola virus and a RNA extraction solution containing magnetic bead, the primer probe sequence is characterized in that an upstream primer is 5'-TGGTGATTTTCCGTTTGATGC-3'; a downstream primer is 5'-AAACGAGC TTGAGCCACTGAAT-3'; and a probe is 5'-FAM-TTGAAATCACAGCATCGTTGGCATC-BHQ13'. The kit has the advantages of rapid operation, simple method, high detection sensitivity (50 copie / ml), and wide detection scope; and can distinguish concrete types of ebola virus. The kit is used for rapidly detecting ebola virus nucleic acid in serum, blood plasma and urine samples and providing reliable experiment basis for diagnosis of ebola virus infection.
Owner:SANSURE BIOTECH INC
Compositions and methods for treating ebola virus infection
InactiveUS20110217328A1Inhibits and treat infectionReduce severitySsRNA viruses negative-senseViral antigen ingredientsViral vectorViral infection
The compositions and methods of the invention described herein provide treatments against Ebola virus infection by expressing gene(s) from the Ivory Coast ebolavirus (ICEBOV) species in a recombinant viral vector.
Owner:TRUSTEES OF BOSTON UNIV +1
Amino acid compositions for the treatment of porcine epidemic diarrhea
Owner:ENTRINSIC LLC +1
Marker genes related with Ebola virus infection and applications thereof
InactiveCN106544441AHigh expressionMicrobiological testing/measurementMicroorganism based processesPTPRK geneFhit gene
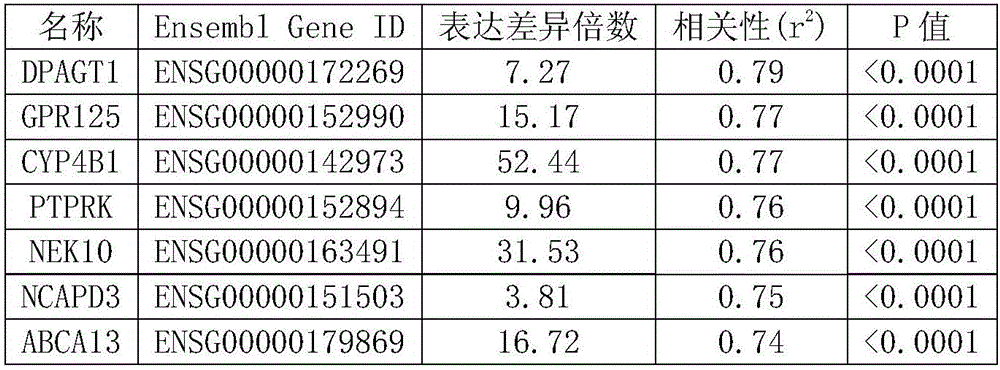
The invention discloses marker genes related with Ebola virus infection and applications thereof. The disclosed marker genes related with Ebola virus infection in blood are 10 following genes: DPAGT1 gene, GPR125 gene, GYP4B1 gene, PTPRK gene, NEK10 gene, NCAPD3 gene, ABCA13 gene, DNAH11 gene, KDELC1 gene, and MTA3 gene. The expression amount of the 10 genes in EBOV infected patients is obviously increased, compared with non-EBOV infected patients, and thus the 10 molecules can be used as biological molecular markers applied to the clinical diagnosis of EBOV infected patients.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Anti-tsg101 antibodies and their uses for treatment of viral infections
InactiveCN101641106AAntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsViral infectionC-terminus
The present invention provides antibodies that bind to the C-terminal region of TSG101. The invention also provides methods of using the TSG101 antibodies for the treatment of viral infections, including HIV and Ebola virus infection.
Owner:ELI LILLY & CO
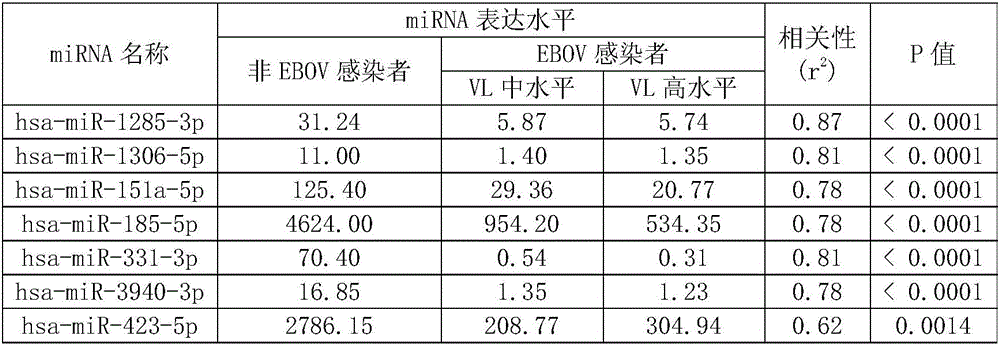
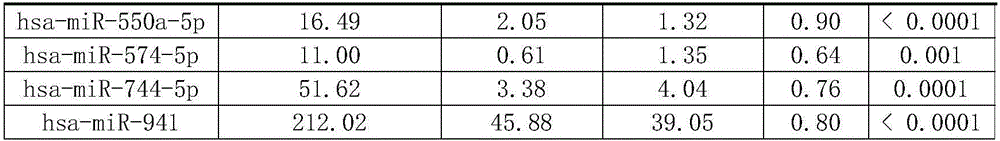
Characteristic miRNAs in Ebola virus infected blood and application of characteristic miRNAs
InactiveCN106636464AMicrobiological testing/measurementDNA/RNA fragmentationClinical diagnosisEbola virus infection
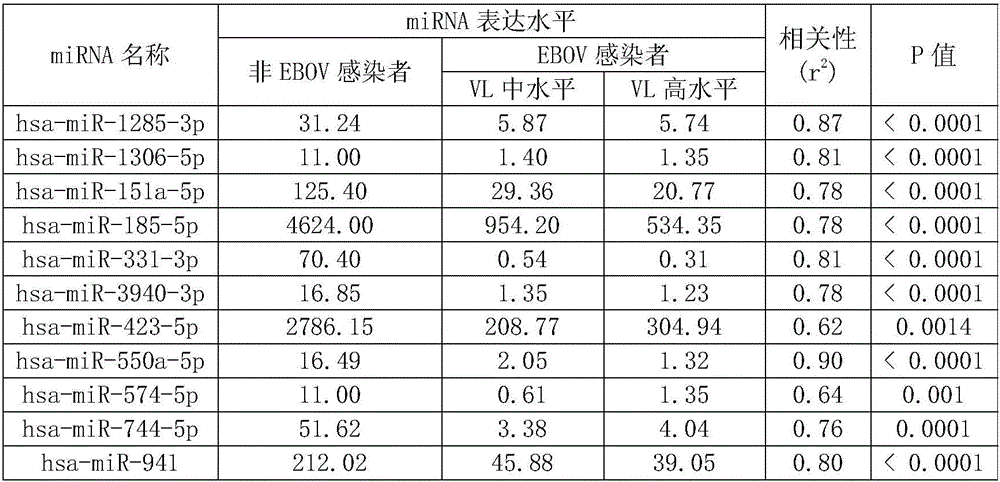
The invention discloses characteristic miRNAs in Ebola virus infected blood and an application of the characteristic miRNAs. The miRNAs in blood related to Ebola virus infection provided by the invention include hsa miR 151a 5p, hsa miR 744 5p, hsa miR 423 5p, hsa miR 331 3p, hsa miR 1306 5p, hsa miR 1285 3p, hsa miR 185 5p, hsa miR 550a 5p, hsa miR 3940 3p, hsa miR 574 5p and hsa miR 941; and the eleven miRNAs, as biological molecular markers, are applicable to clinical diagnosis of EBOV infection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Recombinant antibodies that recongnize the c-terminal domains of ebola virus nucleoprotein
ActiveUS20180334494A1SsRNA viruses negative-senseMicrobiological testing/measurementZaire Ebola VirusProtein antigen
This disclosure is directed to compositions and methods for utilizing the boundaries of the C-terminal domains of Nucleoprotein from Zaire Ebola virus as highly stable recombinant protein antigens to generate antibodies for diagnosis and treatment of Ebola virus infection.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Antibodies specific to glycoprotein (GP) of ebolavirus and uses for the treatment and diagnosis of ebola virus infection
InactiveUS20180016322A1Reduce infectionStrong neutralization abilityBiological material analysisImmunoglobulins against virusesViral infectionSpecific antibody
The present invention relates to antibodies or fragments thereof that specifically bind to glycoprotein (GP) of Ebola virus, and to their use for treating and diagnosing Ebola virus disease.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +3
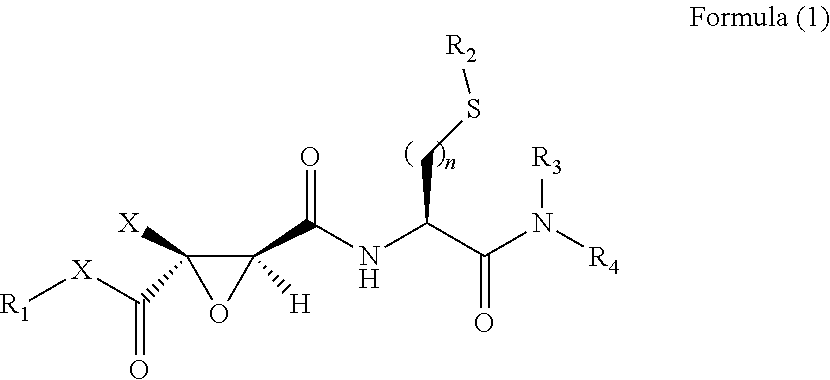
2,3-Epoxy Succinyl Derivative, Preparation Method and Use Thereof
ActiveUS20190062290A1Strong inhibitory activityHigh selectivityOrganic active ingredientsOrganic chemistryEpoxyImmunologic disorders
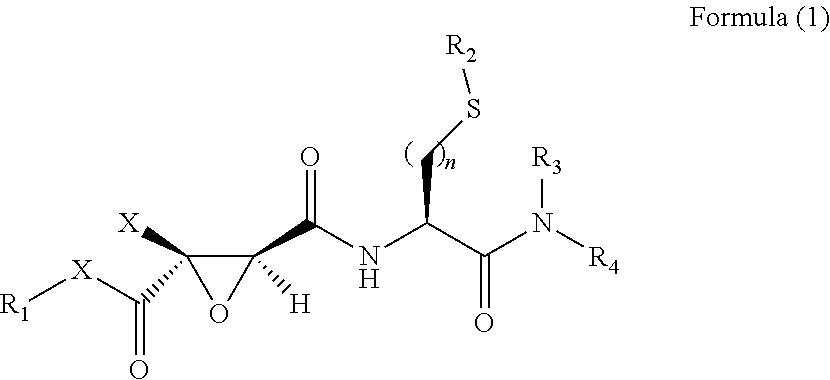
The present invention relates to a 2,3-epoxy succinyl derivative, a preparation method and a use thereof, in particular, the present invention relates to a compound represented by Formula (1), a racemate or an optical isomer thereof, a solvate thereof, or a pharmaceutically acceptable salt thereof. The compound according to the present invention has good inhibitory activity and / or selectivity against cathepsin, especially Cathepsin B, can be used in the treatment of multiple diseases associated with cathepsin, for example, osteoporosis, rheumatoid arthritis and osteoarthritis that are associated with Cathepsin K, Ebola virus infection, a degenerative disease and an autoimmune disease that are associated with Cathepsin L, S, especially Cathepsin B-related tumor diseases, such as gastric cancer, cervical cancer, lung cancer, breast cancer, prostate cancer, bladder cancer, colon cancer, neuroglioma, and melanoma.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
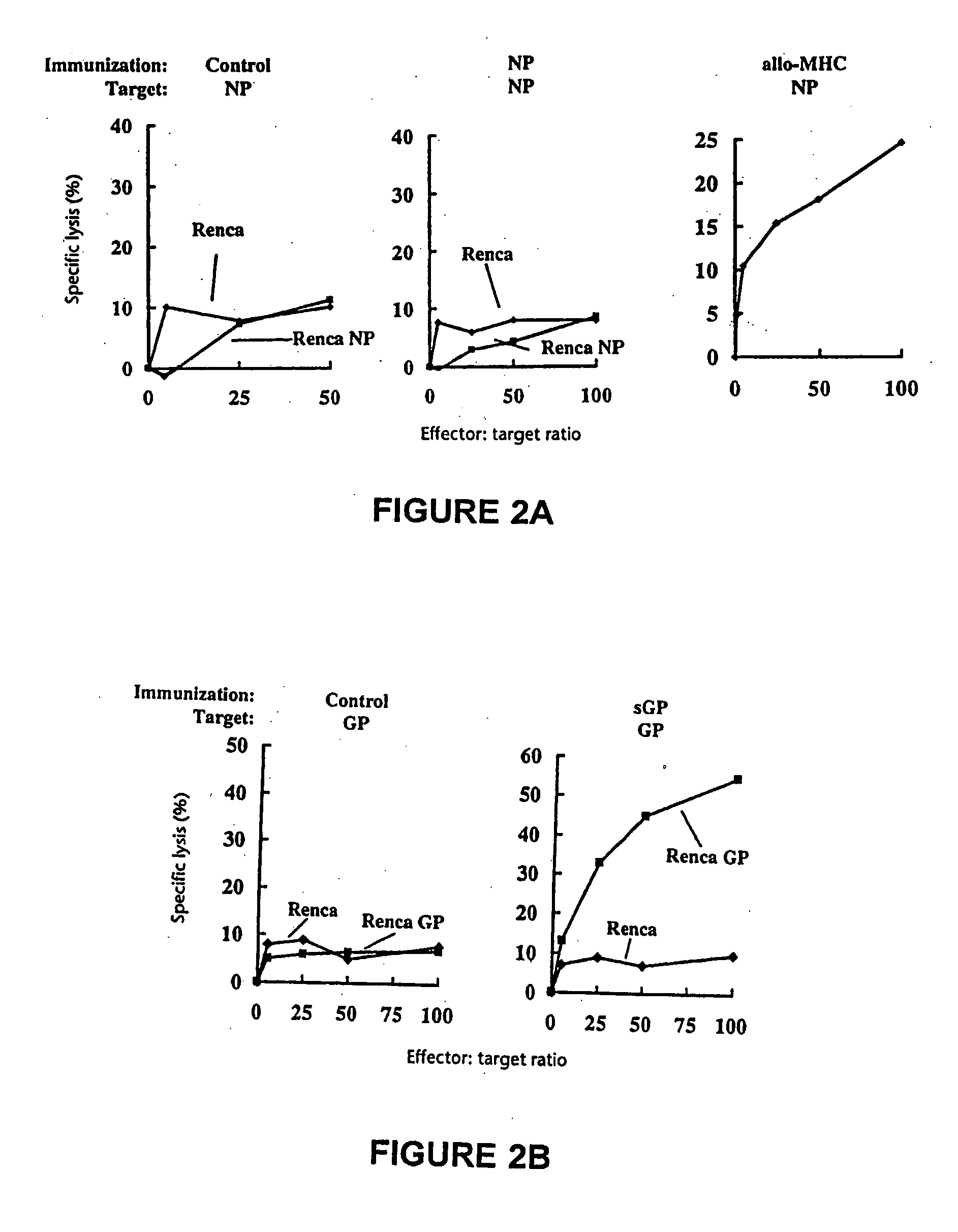
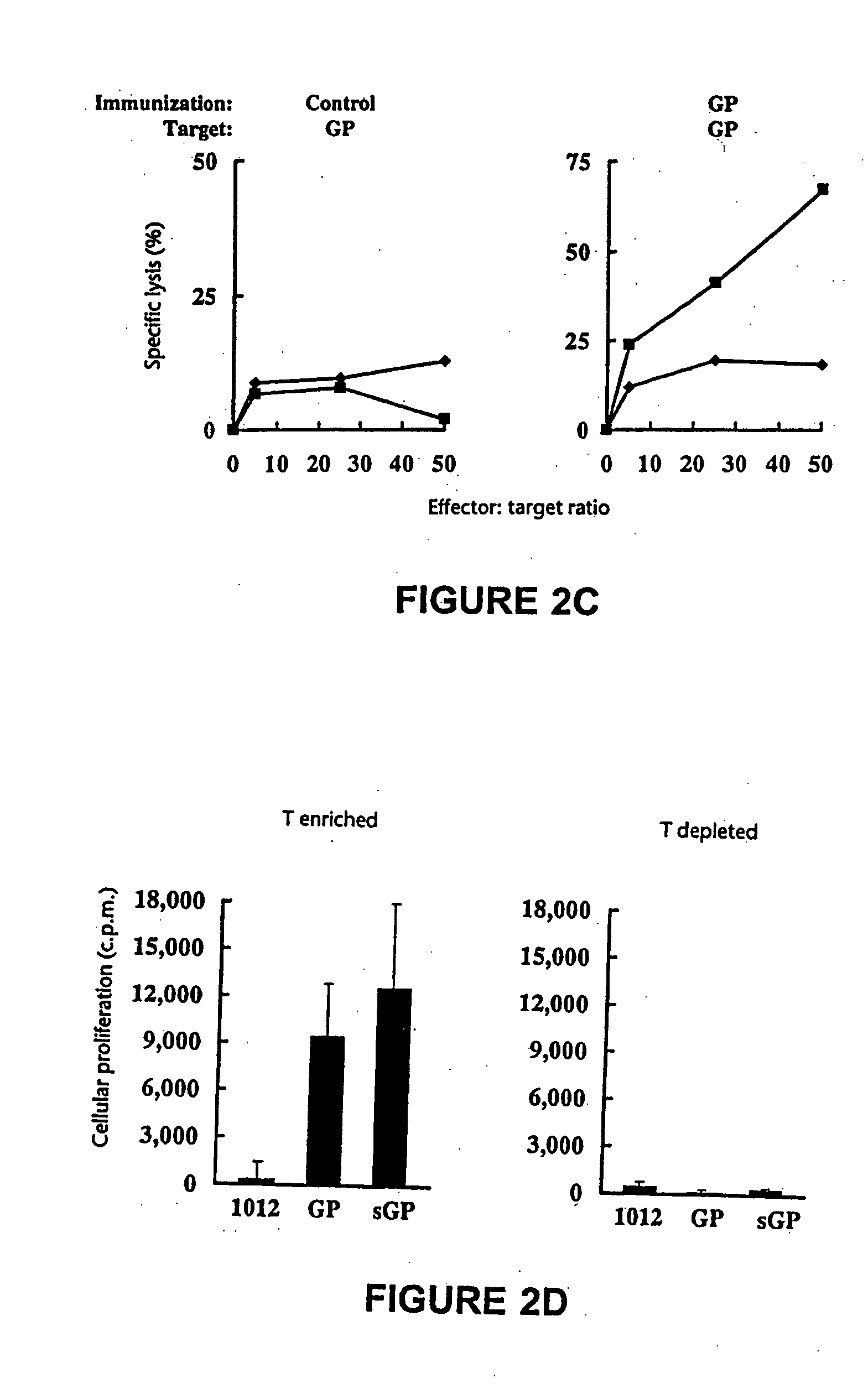
Immunization for Ebola virus infection
InactiveUS20050281844A1SsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinDisease cause
Ebola virus vaccines comprising nucleic acid molecules encoding Ebola viral proteins are provided. In one embodiment, the nucleic acid molecule encodes the transmembrane form of the viral glycoprotein (GP). In another embodiment, the nucleic acid molecule encodes the secreted form of the viral glycoprotein (sGP). In yet another embodiment, the nucleic acid molecule encodes the viral nucleoprotein (NP). Methods for immunizing a subject against disease caused by infection with Ebola virus are also provided.
Owner:NABEL GARY +1
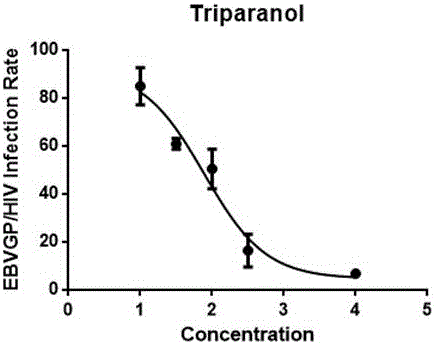
Application of triparanol to resisting Ebola virus infection
The invention provides an application of triparanol to resisting Ebola virus infection, and particularly relates to an application of triparanol to blocking Ebola invasion infection. A theoretical basis is provided for developing an antiviral combined drug taking the triparanol as a main component, and technical support is provided for prevention and control of viral emerging infectious diseases.
Owner:SUZHOU INST OF SYST MEDICINE
Amino acid compositions for the treatment of symptoms of disease
The subject invention provides therapeutic compositions, and uses thereof for the treatment or amelioration of symptoms of a disease selected from the group consisting of: Ebola virus infection, HIV infection, ataxia, environmental enteropathy, cancer, hangover, inflammatory disease, and porcine epidemic diarrhea. In preferred embodiments, the composition includes a combination of one or more amino acids selected from the group comprising lysine, aspartic acid, glycine, isoleucine, threonine, tyrosine, valine, tryptophan, asparagine and / or serine.
Owner:ENTRINSIC LLC +1
Application of telithromycin in anti-ebola-virus infection
ActiveCN109700823AClear securityDrug metabolism properties are clearOrganic active ingredientsAntiviralsAzithromycinAnti virus
The invention discloses application of azithromycin and telithromycin in anti-ebola-virus infection, including application of azithromycin or / and telithromycin in prevention or treatment of ebola virus infection or combined application of azithromycin or / and telithromycin with other anti-virus drugs.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
A nanobody that neutralizes Ebola virus
ActiveCN106188286BGood tissue permeabilityReduce manufacturing costMaterial nanotechnologyBiological material analysisEpitopeNucleotide
The invention provides a nanometer antibody ebo7c2 for neutralizing Ebola viruses and application of the nanometer antibody to preparing medicines for preventing or treating Ebola virus infection. Amino acid sequences of the nanometer antibody are shown as SEQ ID NO:1, and nucleotide sequences for genes for encoding the nanometer antibody ebo7c2 are shown as SEQ ID NO:2. The nanometer antibody and the application have the advantages that CH2 structural domains m01s of human antibodies IgG1 are used as skeletons of the nanometer antibody, and amino acid of the nanometer antibody is different from amino acid sequences of the CH2 structural domains m01s in three loop zones; the nanometer antibody ebo7c2 can be bound with envelope proteins of the Ebola viruses; the nanometer antibody ebo7c2 has small molecular weights and is excellent in tissue permeability and ability of being bound with antigen epitopes with steric hindrance effects; the nanometer antibody is provided with the m01s skeletons and can be bound with FcRn, and the plasma half-life of the nanometer antibody can possibly reach 10 hours; the nanometer antibody ebo7c2 can be expressed in prokaryotic expression systems and is low in production cost and short in cycle.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
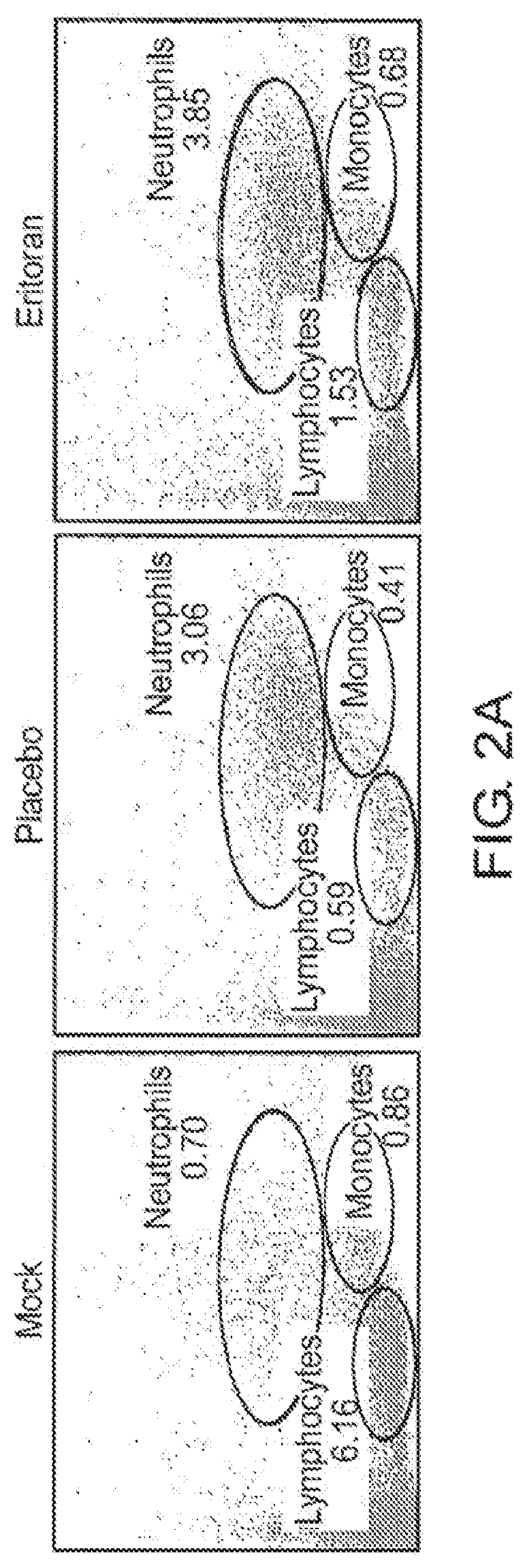
Method of use of eritoran as a TLR4 antagonist for treatment of ebola and Marburg disease
ActiveUS11185554B2Decrease in viral titerOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical medicineMarburg Disease
The present invention is directed to methods for treating ebola virus infections or Marburg virus infections comprising administering to a subject an effective amount of a compound of formula (I) or a pharmaceutically acceptable salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD +1
Inhibitor for Ebola virus
InactiveCN107789344ASignificantly interferes with the infection processInterfere with the infection processOrganic active ingredientsAntiviralsViral infectionEbola virus infection
The invention belongs to the technical field of medicines. In particular, the invention relates to application of a bicyclic amine compound having a structure shown by formula (I) in prevention and / ortreatment of Ebola virus infections or diseases (e.g., Ebola hemorrhagic fever) caused by the Ebola virus infections. The present application also relates to application of a bicyclic amine compoundhaving a structure shown by formula (I) in preparation of pharmaceutical compositions for preventing and / or treating Ebola virus infections or the diseases (e.g., Ebola hemorrhagic fever) caused by the Ebola virus infections.
Owner:NAT INST FOR FOOD & DRUG CONTROL
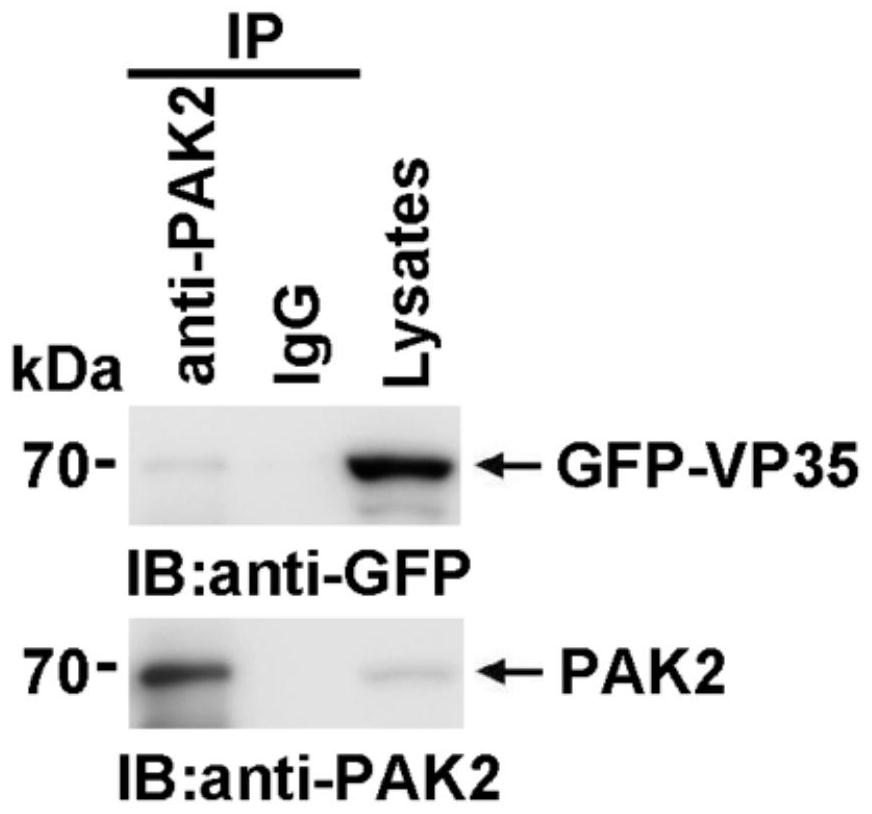
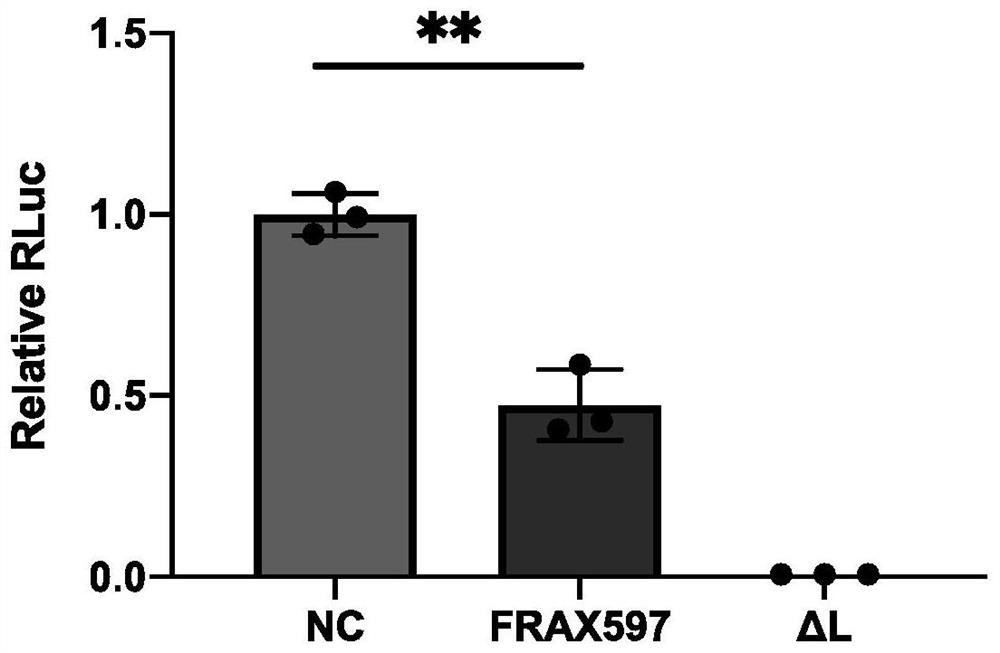
Application of FRAX597 compound in preparation of medicine for treating Ebola virus disease
ActiveCN114869889AReduce duplicationPrevent proliferationOrganic active ingredientsAntiviralsThreonineBULK ACTIVE INGREDIENT
The invention relates to application of an FRAX597 compound in preparation of a medicine for preventing Ebola virus infection or treating Ebola virus diseases. Researches show that after cells are treated with the FRAX597 inhibitor compound of serine / threonine kinase PAK2 (Gene ID: 5062), replication of the Ebola virus in the cells is remarkably reduced, and proliferation of the Ebola virus is inhibited. CCK8 is used for detecting the cytotoxicity of the FRAX597, and it is proved that the FRAX597 shows extremely low or even negligible cytotoxicity under the condition that the concentration is controlled. Therefore, the FRAX597 can be used as an active ingredient for preparing a candidate medicine for treating and resisting the Ebola virus disease.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com