Monoclonal antibody of human anti-Ebola virus envelope glycoprotein, and application thereof
An Ebola virus, monoclonal antibody technology, applied in the field of microbiology and immunology, can solve the problem of insufficient stimulation of Ebola GP membrane fusion process, and achieve the effect of excellent antigen binding activity and excellent neutralizing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Screening and preparation of human monoclonal antibodies against Ebola virus envelope glycoprotein
[0030] 1. Blood Sample Collection
[0031] After obtaining informed consent, 5 mL of blood samples were collected 14 days after the first immunization of recombinant Ebola vaccine clinical trial subjects for subsequent experiments.
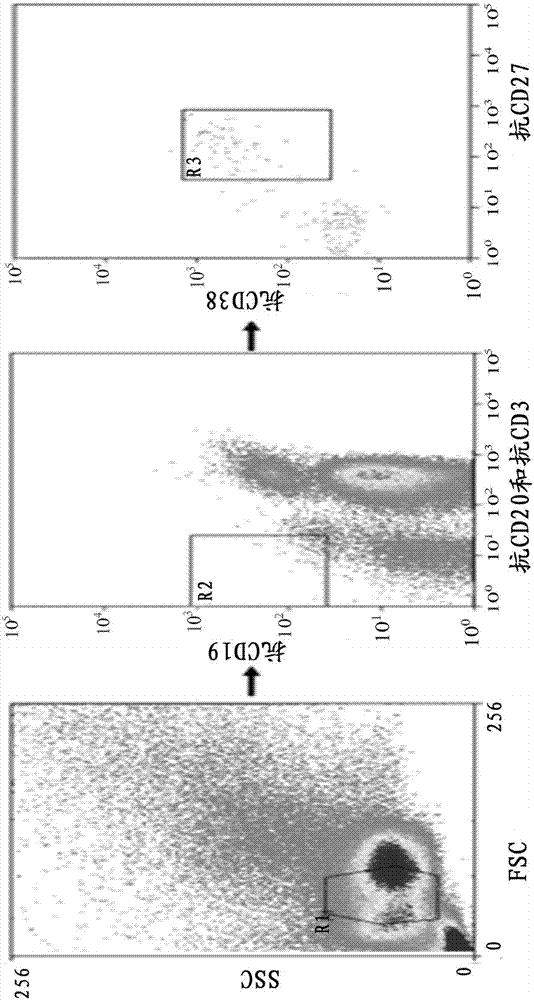
[0032] 2. Sorting Single Cells by Flow Cytometry
[0033]The collected blood samples were separated from PBMC by Ficoll density gradient centrifugation, and the process was as follows:
[0034] 1) Take fresh anticoagulated whole blood and anticoagulate with EDTA. Whole blood was diluted with an equal volume of PBS or 0.09% NaCl.
[0035] 2) Add a certain volume of separation liquid into the centrifuge tube, spread the diluted blood sample above the liquid surface of the separation liquid, and keep the interface between the two liquid surfaces clear. The volume of separation medium, anticoagulated undiluted whole blood, and PBS ...
Embodiment 2
[0120] Example 2: Antigen epitope analysis of neutralizing monoclonal antibody 1B3 binding to GP
[0121] 1. Analysis of the binding effect between 1B3 and GP and its truncated antigen
[0122] Binding of mAbs to truncated antigens was detected by ELISA. Construct the expression plasmids of three GP truncated antigens GP1 (33-500aa), GPdmucin (33-310aa, 463-632aa), sGP (33-294aa), express the above-mentioned three truncated antigens with Expi293 mammalian cell system, and use the package The lectin on the ELISA plate captured the full-length and truncated antigens of GP respectively, and identified the binding activity of 17 specific antibodies to GP and its truncated antigens. Zmapp optimized strain MIL77-1 / 2 / 3 and anthrax-specific monoclonal antibody 8A7 were used as positive and negative controls, respectively.
[0123] Result: if Figure 5 , MIL77-3 can bind to all four forms of GP, and MIL77-1 / 2 can only bind to the full length of GP and GPdmucin, which is consistent w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com