Anti-tsg101 antibodies and their uses for treatment of viral infections
A-TSG101, a virus infection technology, applied in the detection of virus-infected cells and the treatment of virus infections including HIV infection, can solve the problem of drug resistance weakening the therapeutic potential of virus fusion inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Example 1: Preparation and use of anti-TSG101 polyclonal antibody
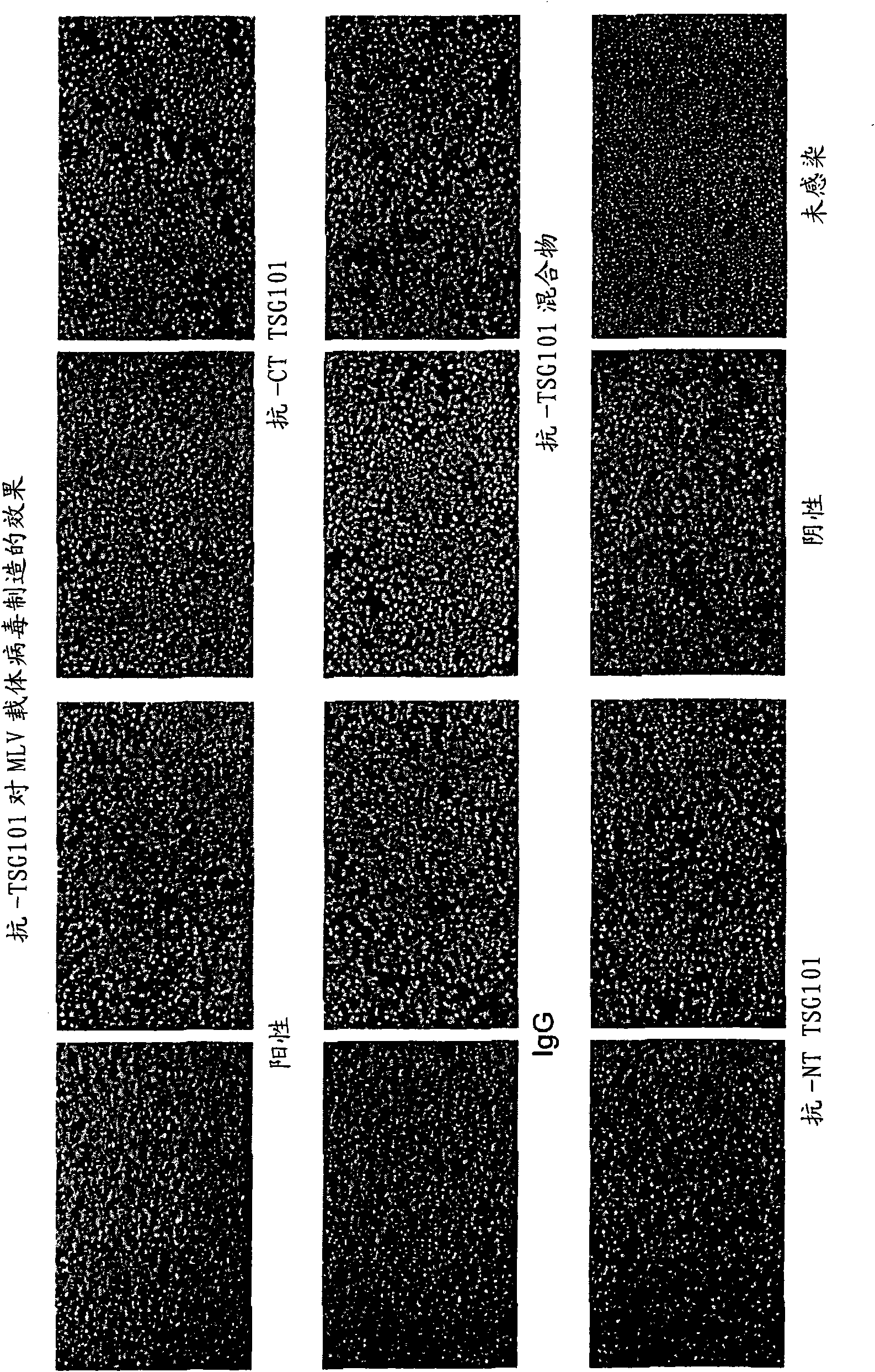
[0228] To determine the effect of anti-TSG101 antibodies on viral infection, a retroviral infection assay was developed. Murine leukemia virus (MLV)-derived vectors including the E. coli lacZ gene expressed from the long terminal repeat (LTR) promoter (pBMN-Z-INeo) were transfected with facultative murine leukemia from 293 cells (Phoenix A, ATCC) Retroviral packaging cell lines. Retroviruses produced from Phoenix A helper cells were harvested and used to infect mouse N2A cells (ATCC). Anti-TSG101 antibody was added to 293 helper cells 24 hours after MLV vector transfection. The effect of TSG101 antibody on virus production was determined by the efficiency of viral supernatants to infect target cells (N2A). Infection of N2A cells was subsequently determined by cell staining for 3-galactosidase activity (positive cells stained blue, as figure 2 shown by dark dots in ).
[0229] Typically, phoenix ...
Embodiment 2
[0231] Example 2: Localization of TSG101 on the cell surface during virus budding
[0232] This example shows that the domain of TSG101 is exposed on the cell surface during HIV shedding and anti-TSG101 antibodies inhibit HIV shedding and infection.
[0233] Localization of TSG101 during viral shedding
[0234] In order to prove that TSG101 is actively involved in the release of the virus at the plasma membrane, the expression vector of the GFP-TSG101 fusion protein was constructed and transfected into Phoenix cells that actively produce M-MuLV virus (retroviral helper cell line developed by Nolan et al. ). 24 hours after transfection, the GFP-TSG101 fusion protein pathway was observed under a confocal microscope (Ultraview, Perkin-Elmer). Figure 3A ~E shows images of the time course of GFP-TSG101 protein translocation from the cytoplasm to the cell surface and then "budding" out of virus-producing cells.
[0235] Cell surface localization of TSG101 during HIV buddin...
Embodiment 3
[0246] Example 3: Effect of Anti-TSG101 Monoclonal Antibody on HIV Infection
[0247] Production of anti-TSG101 monoclonal antibody (mab)
[0248] A peptide consisting of 19 amino acids at the C-terminus of TSG101 (qlralmqkarktaglsdly, SEQ ID NO: 3) was synthesized, paired with keyhole limpet hemocyanin (KLH) and used to immunize 5 mice. Serum samples from each mouse were tested against BSA-conjugated C-terminal TSG101 peptide by ELISA. Mice with the highest antibody titers were killed and spleens fused with myeloma cells to generate a pool of hybridomas. PE-8 was one of a pool of hybridomas containing two hybridomas producing a mab that recognized the immunizing peptide (SEQ ID NO: 3). Pool PE-8 is also referred to as antibody pool PE-8.
[0249] Anti-TSG101mab inhibits wild-type and drug-resistant HIV
[0250] Briefly, wild-type and drug-resistant HIV expression vectors were transfected into HEK293 cells. 24 hours after transfection, transfected cells were treated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com