Hypersensitivity Epstein-Barr (EB) virus fluorescence quantitative polymerase chain reaction (PCR) kit for locked nucleotide acid (LNA) and detection method and application thereof
A fluorescence quantitative, EB virus technology, applied in the field of molecular biology, to achieve the effect of short detection time, reduced result deviation, and large sample size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
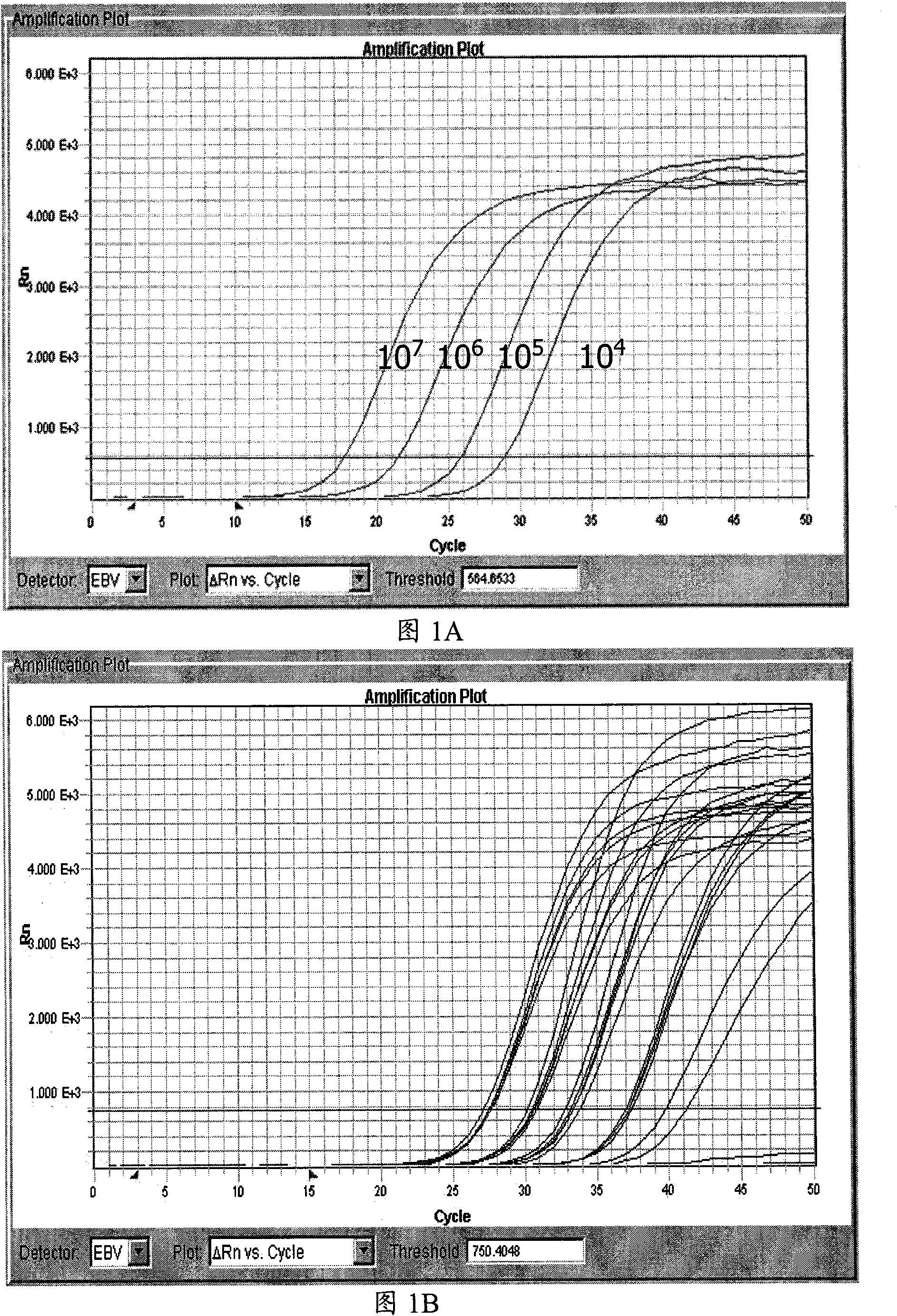
[0038] Example 1: Reproducibility experiment of fluorescent quantitative PCR kit detecting EBV-DNA in plasma of patients with nasopharyngeal carcinoma
[0039] The Epstein-Barr virus fluorescence quantitative PCR kit of locked nucleotide (LNA) sensitization of the present invention comprises LNA (locked nucleotide)-TaqMan fluorescence quantitative reaction solution, positive control sample, wherein, LNA-TaqMan fluorescence quantitative reaction solution comprises:
[0040] Forward primer: 5'-AAT TTT TTC TGC TAA GCC CAA CA-3';
[0041] Reverse primer: 5'-ACG GGT GGG TGT GTG TAG TGT-3';
[0042] LNA-TanMan fluorescent probe: 5'-FAM-CCA CCA CAC CCA GGC-MGB3'.
[0043] The positive control sample is the plasmid DNA containing the Epstein-Barr virus genome fragment.
[0044] To use this kit to detect EBV-DNA, proceed as follows:
[0045] (1) Preparation of fluorescent quantitative nucleic acid amplification reaction system: add 0.15 μl (20 μM) of forward primer, 0.15 μl (20 μM) ...
Embodiment 2
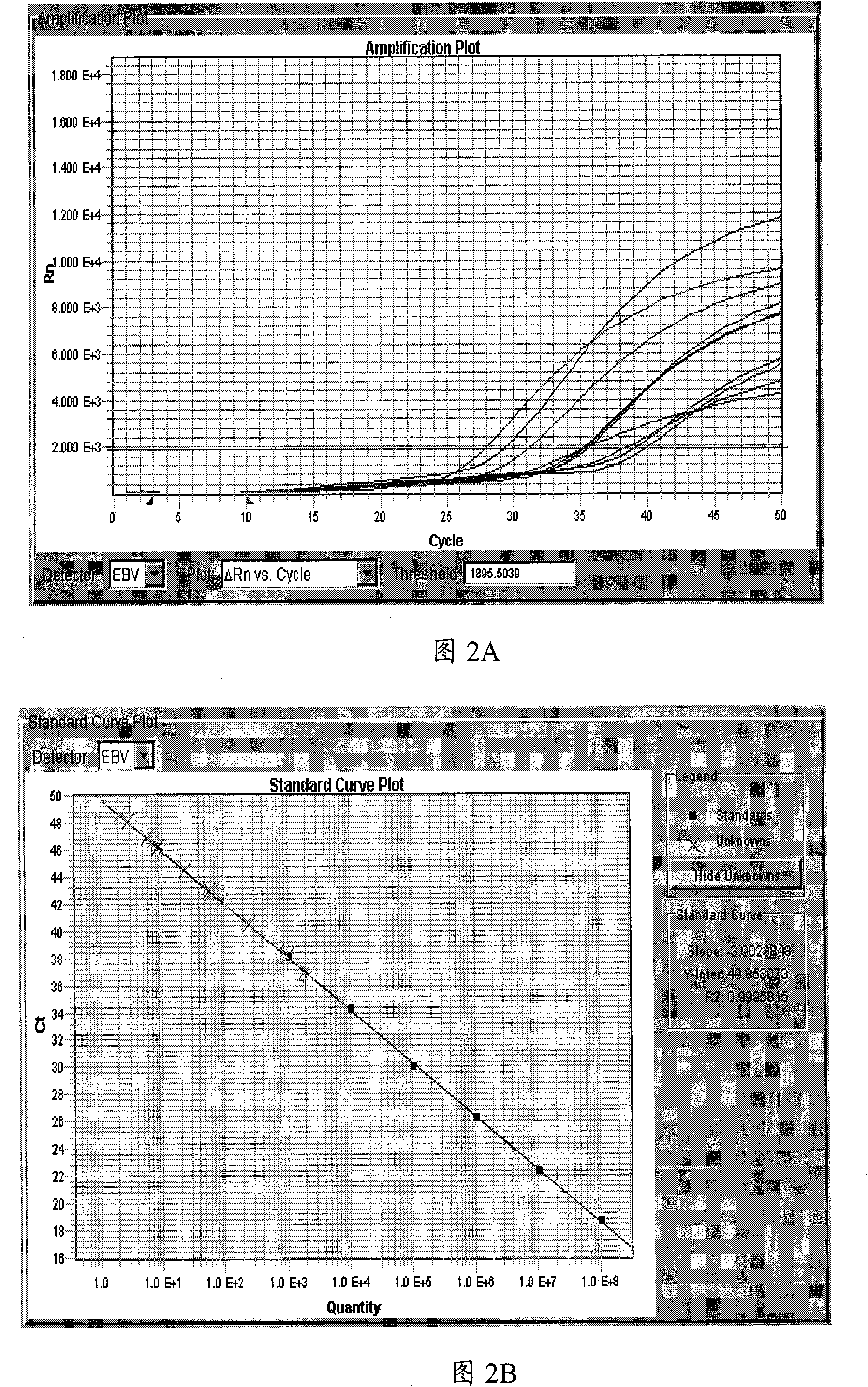
[0049] Embodiment 2: The EBV-DNA fluorescent quantitative PCR kit of the present invention detects and detects the plasma EBV-DNA concentration of nasopharyngeal carcinoma patients
[0050] Fig. 2 is a preferred result diagram of detecting plasma EBV-DNA concentration of nasopharyngeal carcinoma patients with the EBV-DNA fluorescent quantitative PCR kit of the present invention, the method is the same as that described in Example 1. in, Figure 2A It shows that the sample amplification curve is clear, and the negative control has no amplification; Figure 2B Standard curve for comparison. The results of PCR amplification were analyzed automatically with the automatic analysis software SDS (Version 2.0). The quantitative results of plasma EBV-DNA were converted according to the formula: C=Q×(V DNA / V PCR )×(1 / V EXT ). Wherein C is the concentration (copy / ml) of EBV-DNA in the plasma to be tested, Q is the EBV-DNA concentration that PCR reaction obtains, V DNA Final dilut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com