Miniaturized polypeptide of anti EB Virus tumour, application and preparation method
A technology for Epstein-Barr virus and tumors, applied in the field of miniaturized anti-EB virus tumor polypeptides and its application and preparation, can solve the problems of large immunogenicity, large molecular weight, adverse allergies, etc., and achieve specific targeting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Construction of plasmids expressing anti-tumor polypeptides and preparation of recombinant miniaturized anti-EB virus tumor polypeptides
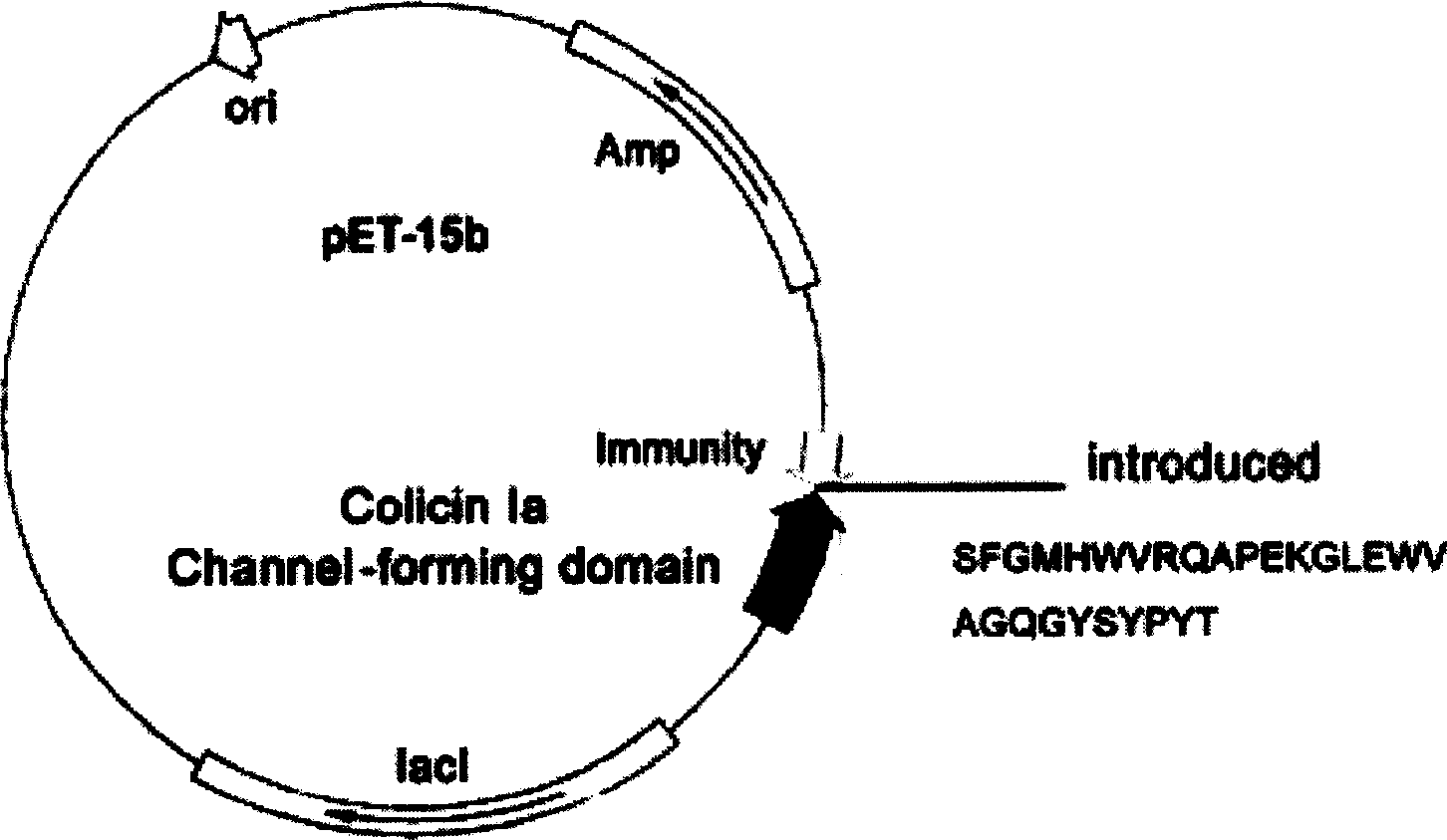
[0029] The original plasmid is the pET-15b commercial plasmid loaded with colistin Ia ion channel domain and Immunity protein gene (plasmid size 6.3kb, purchased from Novagen, the above-mentioned genes were loaded by our laboratory), and double-stranded oligonucleotide Point mutation technology (QuickChange TM Kit, Strategene Company) inserts the gene (the nucleotide sequence described in SEQID NO.3, 5, 7, 9, 11 and 13 in the sequence listing) of the coding guide polypeptide into the I626 site of the colistin Ia channel domain gene Finally, six recombinant plasmids pCHCEBCH1 to pCHCEBCH6 (such as Figure 1~6 shown). The recombinant plasmid was transfected into E.coli BL21(DE3)ply S engineering bacteria to prepare polypeptides, and six miniaturized anti-EB virus tumor polypeptides were obtained, the amino acid sequences o...
Embodiment 2
[0067] Example 2 Construction of plasmids expressing anti-tumor polypeptides and preparation of recombinant miniaturized anti-EB virus tumor polypeptides
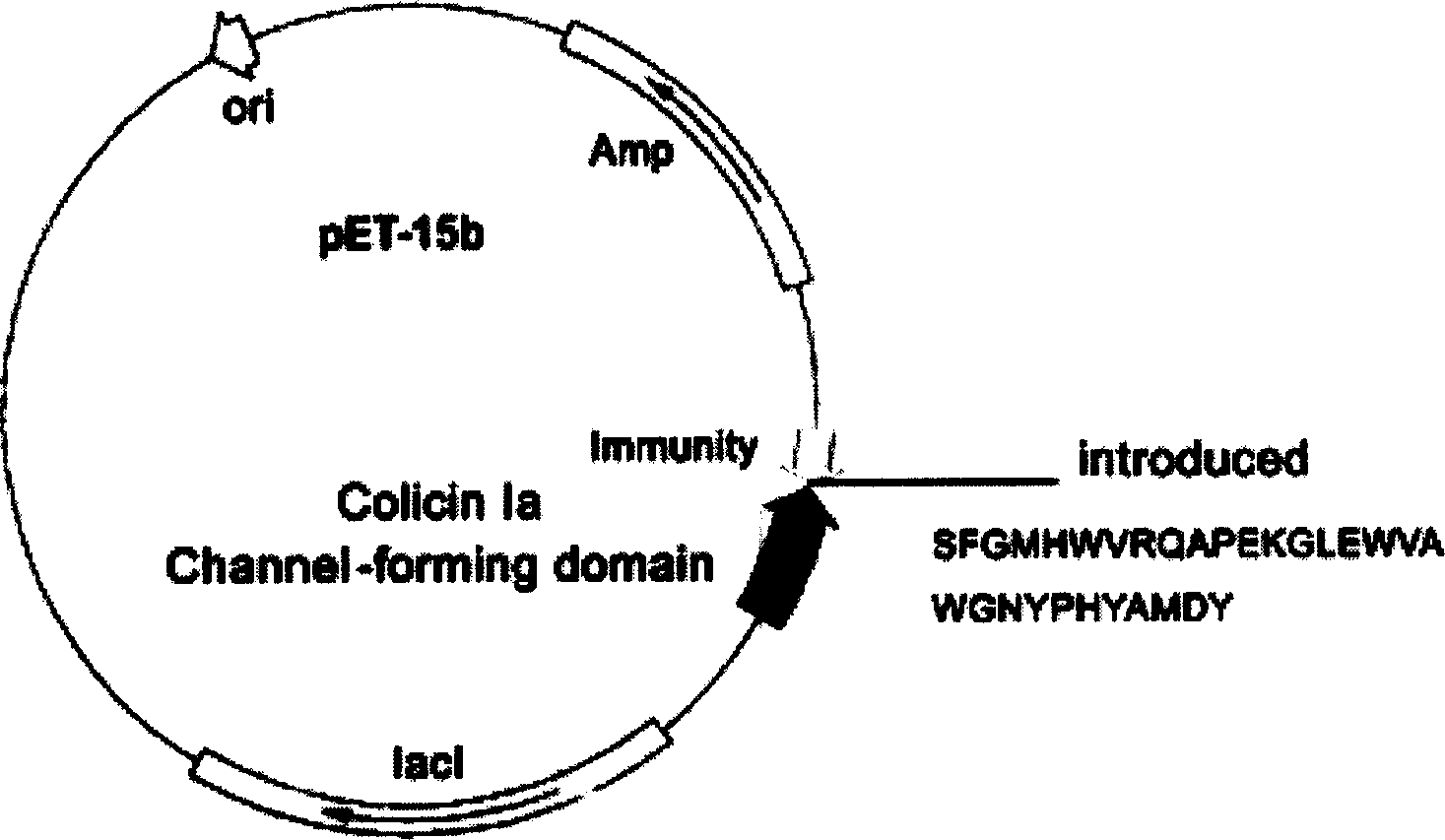
[0068] The original plasmid was the pET-15b commercial plasmid (6.3 kb in size, purchased from Novagen, the above-mentioned gene was loaded by our laboratory) loaded with the colistin A ion channel domain and the Immunity protein gene. Point mutation technology (QuickChange TM Kit, Strategene Company) inserted the gene encoding the guide polypeptide (the nucleotide sequence described in SEQID NO.3 in the sequence listing) into the H592 site of the colicin A ion channel domain gene, and prepared the anti-tumor polypeptide A recombinant plasmid pCHCEBCH8 (such as Figure 8 shown). The recombinant plasmid was transfected into the E.coli BL21 (DE3) ply S engineering bacterium to prepare the polypeptide, and obtained the miniaturized anti-EB virus tumor polypeptide described in SEQ ID NO 30 in the sequence listing (referred t...
Embodiment 3
[0086] Example 3 Construction of plasmids expressing anti-tumor polypeptides and preparation of recombinant miniaturized anti-EB virus tumor polypeptides
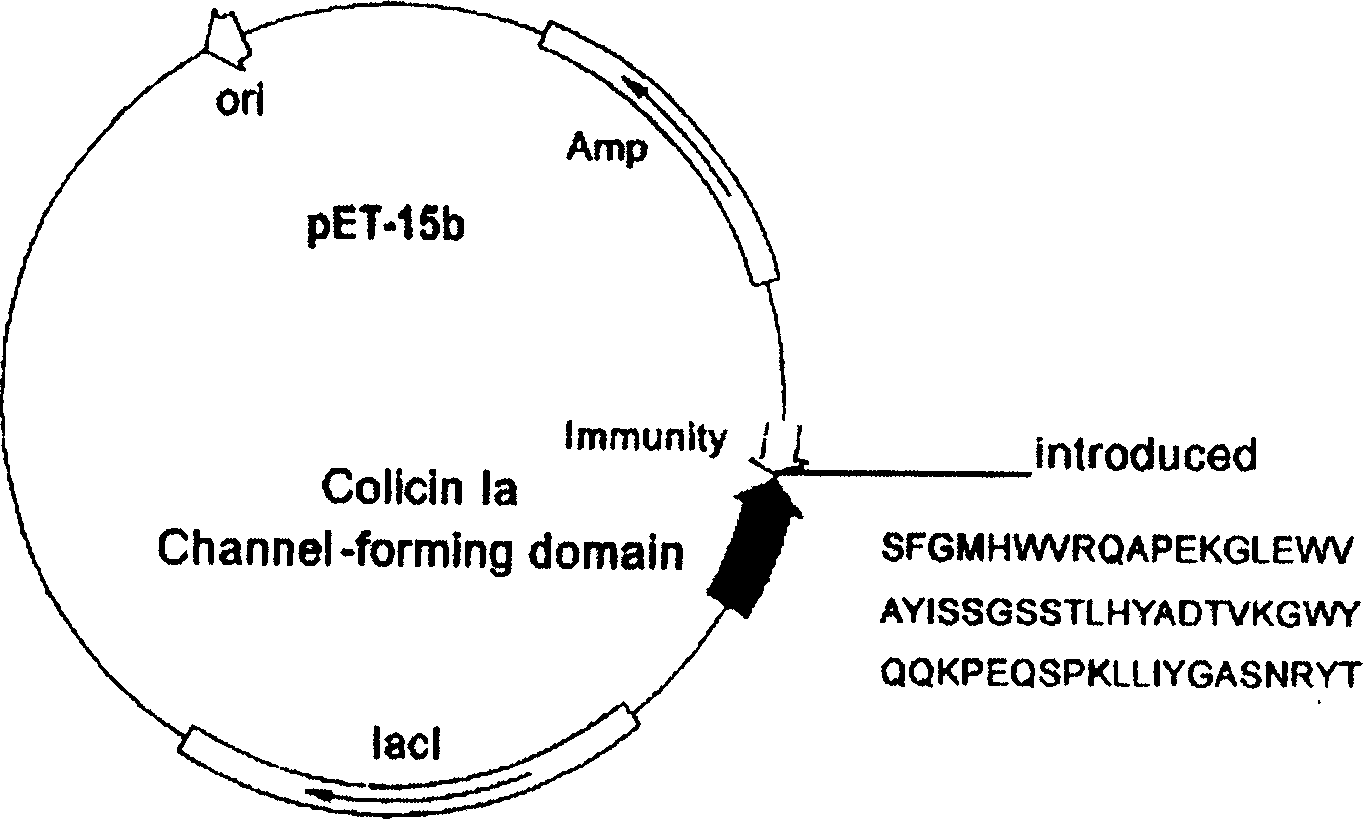
[0087] The original plasmid is the pET-15b commercial plasmid loaded with the colistin Ia ion channel domain and the Immunity protein gene (the plasmid size is 6.3kb, purchased from Novagen, and the above-mentioned genes are loaded by our laboratory). Acid point mutation technology (QuickChange TM Kit, Strategene Company) inserted the gene encoding the guide polypeptide (the nucleotide sequence described in SEQ ID NO.3 in the sequence listing) before the D451 site of the colicin Ia ion channel domain gene, and prepared the anti-tumor polypeptide A recombinant plasmid pCHCEBCH7 (such as Figure 7 shown). The recombinant plasmid was transfected into E.coliBL21 (DE3) ply S engineering bacteria to prepare polypeptides, and obtained the miniaturized anti-EB virus tumor polypeptide described in SEQ ID NO 28 in the sequence lis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com