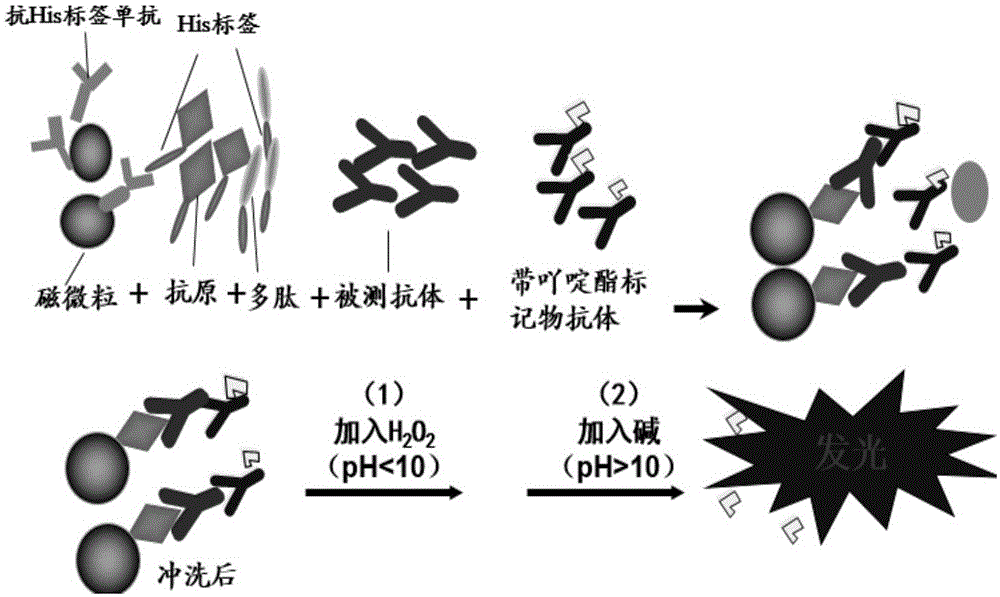
Chemiluminescence kit for detecting EB virus Rta/IgG antibody and use thereof
A chemiluminescence reagent, EB virus technology, applied in the fields of biotechnology, medical immunology and in vitro serological diagnosis, can solve the problems of enhanced background luminescence, limited application and development of sensitivity, elevated measurement background, etc., to simplify steps and shorten the Detection time, easy operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1. Preparation of kit of the present invention
[0059] 1. Preparation of EB virus Rta protein and polypeptide
[0060] Preparation: inoculate the CHO / RTA cell line with the preservation number: CGMCC6955 into the cell culture dish, the complete culture solution of the cells must be supplemented with 0.1mmol / L hypoxanthine and 0.016mmol / L thymine, 10% fetal bovine serum (Hangzhou Sijiqing Bioengineering Co., Ltd.), after the cells reach a density of about 90%, replace with a serum-free medium without aminomethylpterate (MTX), collect the cell culture medium after 1-2 days, and use trichloro Proteins in cell culture fluid were extracted by acetic acid precipitation.
[0061] Purification: Use GE's streptavidinsepharose highperformance to purify the target protein in the cell supernatant. According to the operation manual of BCA-100 protein quantification kit (Beijing Saichi Biology, product number 300001-B), the test is carried out to quantitatively measure ...
Embodiment 2
[0079] Embodiment 2. Utilize the kit detection sample of the present invention
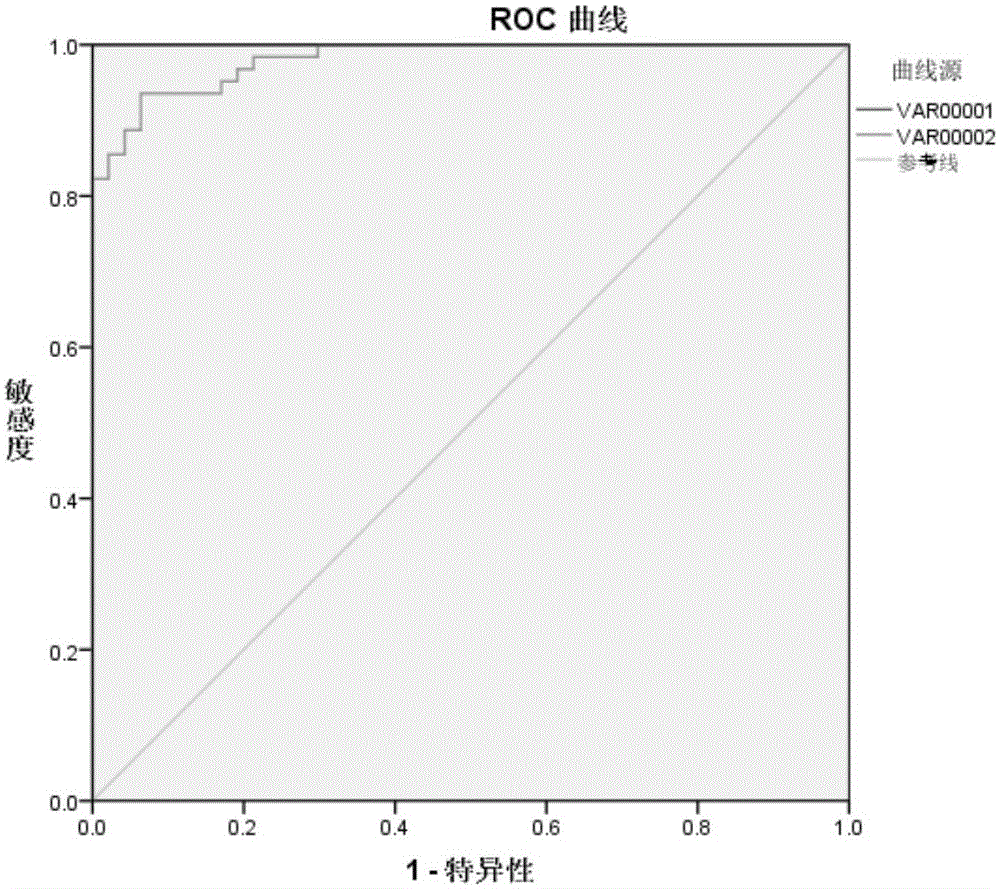
[0080] Detect 45 parts of nasopharyngeal carcinoma samples and 45 parts of healthy check-up samples with kit of the present invention to check: the steps are as follows:
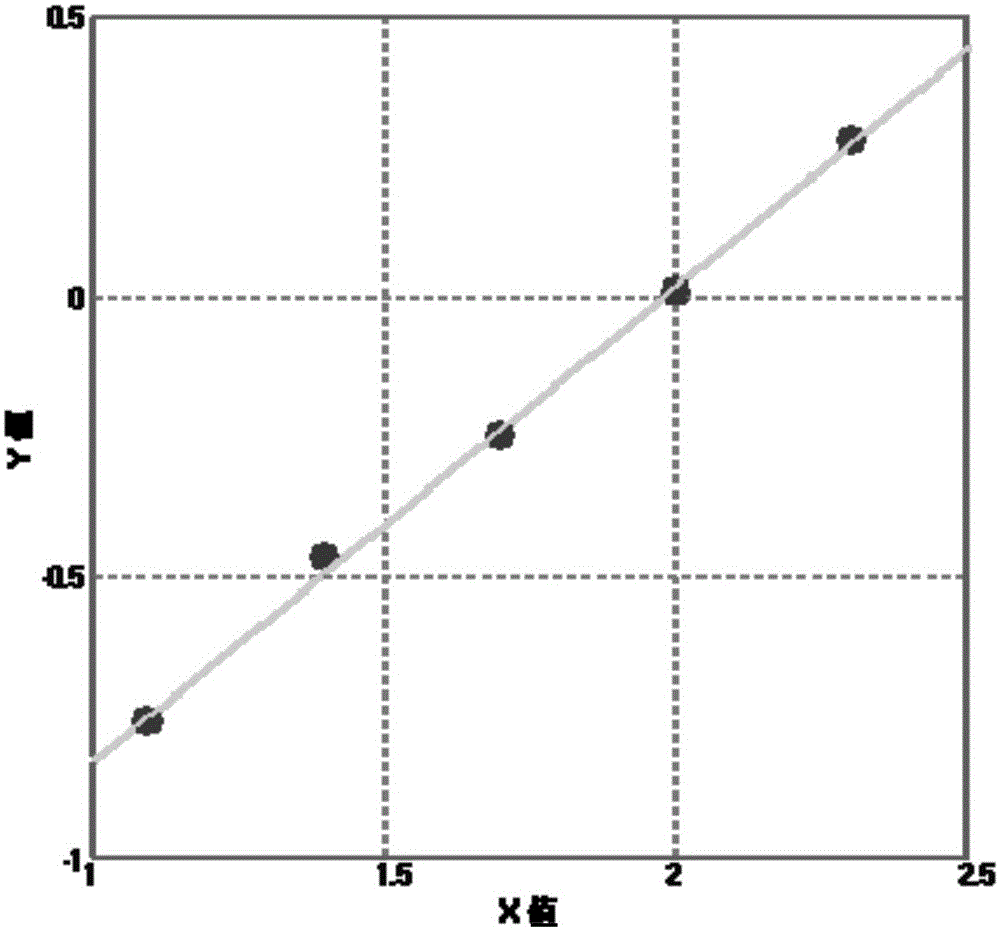
[0081] (1) Obtain standard curve and curve equation,
[0082] The abscissa is the gradient concentration value of the Rta / IgG calibrator, and the ordinate is the fluorescence value obtained by performing the following steps with the gradient concentration of the Rta / IgG calibrator;
[0083] (2) Add working solution 1 and working solution 2 successively on the slide, incubate and dry;
[0084] (3) Add the sample to be tested diluted by the sample diluent, incubate and dry;
[0085] (4) Add working solution 3, incubate and dry;
[0086] (5) After washing with washing solution, place the glass slide under a fluorescence microscope to observe the reading;
[0087] (6) Substitute the fluorescence value into the standard curve to ...
Embodiment 3
[0101] Embodiment 3. Use the test kit prepared by Rta protein as the reaction antigen to detect the comparative experimental data of the same sample
[0102] Using the kit prepared with Rta protein as the reactive antigen to detect 45 nasopharyngeal carcinoma samples and 45 healthy physical examination samples, the test results are as follows:
[0103] Table 4. Detection results of normal human EB virus Rta-IgG content
[0104]
[0105] Table 5. Detection results of EB virus Rta-IgG content in patients with nasopharyngeal carcinoma
[0106]
[0107]
[0108] It can be known from the experimental results that the sensitivity and specificity of the kit prepared using Rta protein as the reactive antigen are 91.2% and 93.2%, both of which are lower than the sensitivity and specificity of the kit of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com