Preparation method of recombinant adeno-associated viruses containing EB virus latent membrane protein 1 and 2 genes and application thereof
A latent membrane protein, Epstein-Barr virus technology, applied in the field of bioengineering, can solve the problems of infection risk of tumor patients and the pathogenicity of adenovirus, and achieve the effects of low toxicity, wide infection spectrum and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
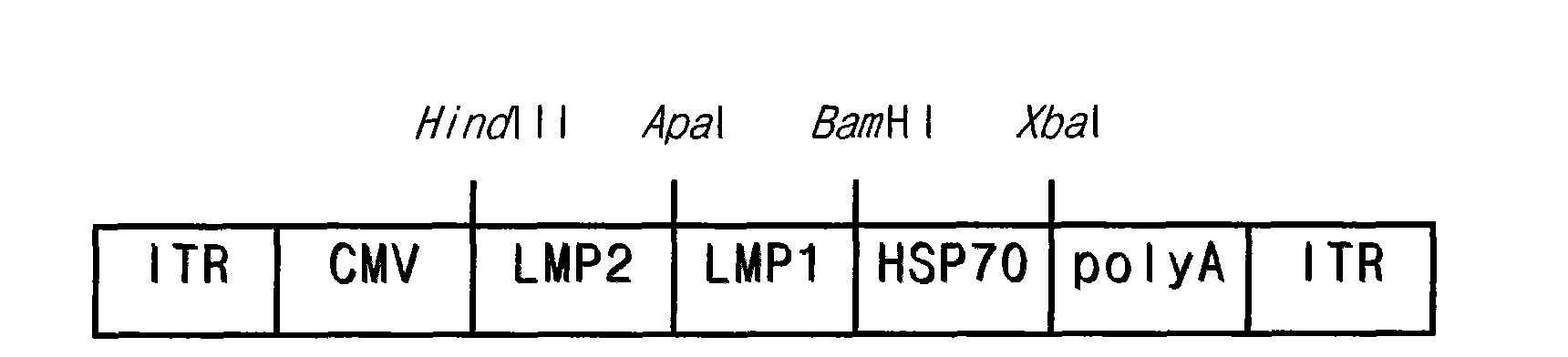
[0034] Example 1: Construction of rAAV-LMP2 / 1-HSP recombinant adeno-associated virus
[0035] 1. Construction of pAAV-LMP2 / 1-HSP recombinant adeno-associated virus vector plasmid
[0036] (1) Preparation of Escherichia coli Competent Cells
[0037] Take out the E.coli DH5α strains stored at -80°C, pick a small amount of bacterial solution with an inoculation loop and streak it on the LB agar plate, and culture it upside down at 37°C overnight; pick a single DH5α colony the next day, and inoculate it in 3ml LB medium ( (without antibiotics), shake overnight at 280rpm on a shaker at 37°C; take 0.5ml of the above bacteria solution and inoculate it into 50ml LB medium the next day, shake at 280-300rpm on a shaker at 37°C until the OD value of the bacteria solution is A 600 Take it out when it reaches 0.4~0.6; centrifuge at 5,000rpm at 4°C for 10min, discard the supernatant, and pre-cool with 20ml CaCl 2 (0.1mol / L) resuspended bacteria, after 30min in ice bath, centrifuge at 5,00...
Embodiment 2
[0071] Embodiment 2: the determination of rAAV-LMP2 / 1-HSP and rAAV-GFP virus titer:
[0072] (1) Probe labeling
[0073] The CMV promoter fragment in the vector was labeled [α- 32 p]dCTP as a probe:
[0074] Dissolve CMV in sterilized TE at a concentration of 25 μg / mL, boil at 100°C for 2 minutes and place on ice immediately;
[0075] Add the following reactants sequentially on ice:
[0076] Component Amount Final Concentration
[0077] 5×labeling buffer 10μl 1×
[0078] dNTPs mixture 2μl 20μM / each
[0079] CMV template 1μl 500ng / ml
[0080]BSA 2μl 400μg / ml
[0081] [α- 32 P]-dCTP (3000Ci / mmol) 5μl 333nM
[0082] Klenow enzyme 1μl 100u / ml
[0083] Sterilized water 29μl
[0084]
[0085] Total volume 50μl
[0086] Gently mix the above reactants and incubate at room temperature for 60 min;
[0087] After boiling at 100°C for 2 minutes, place it on ice immediately;
[0088] Add EDTA to a final concentration of 20...
Embodiment 3
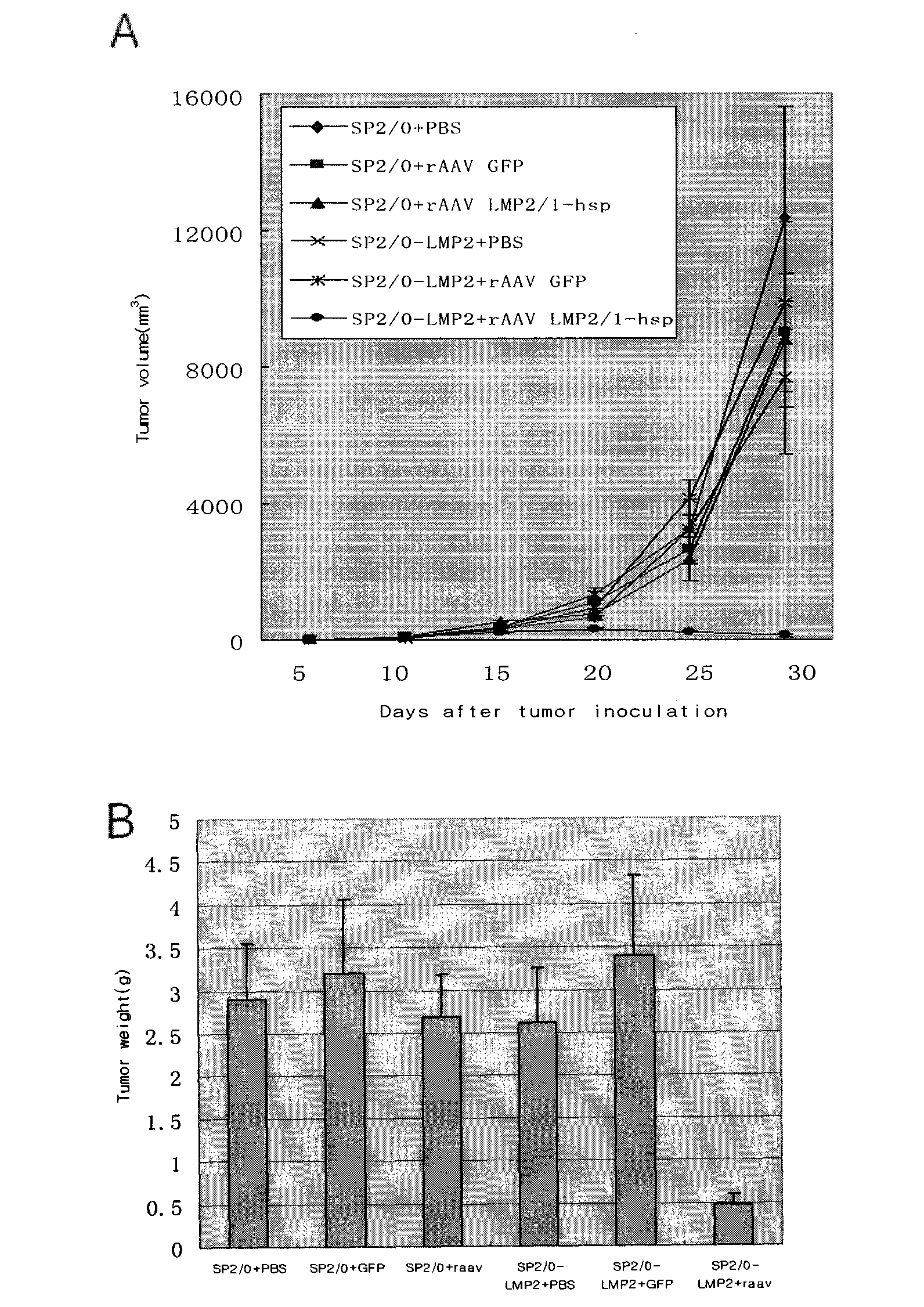
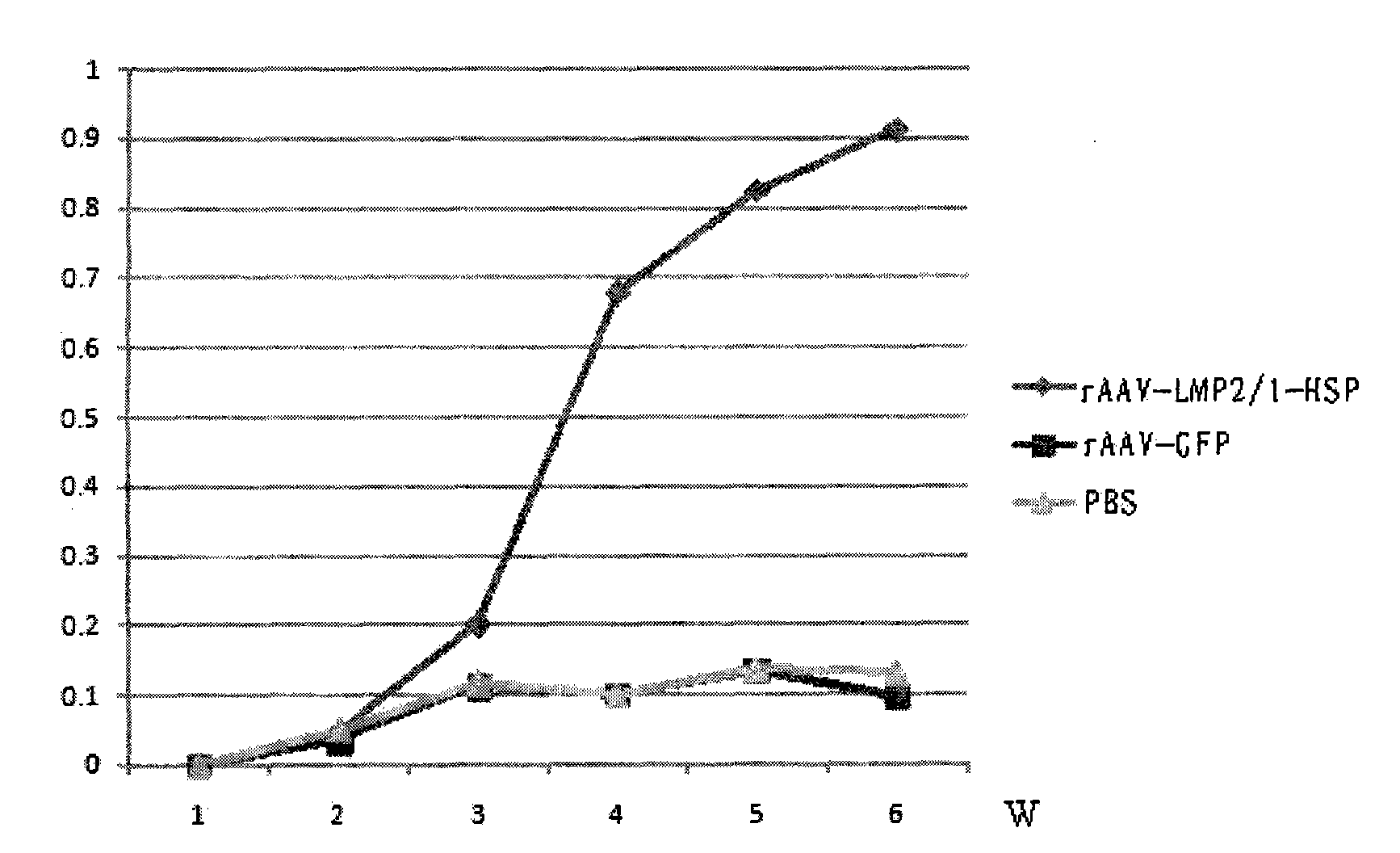
[0107] Example 3: Analysis of immune activity of rAAV-LMP2 / 1-HSP
[0108] BALB / c myeloma cells (SP2 / 0 cells) were routinely cultured. When the cells grew to the mid-log phase, they were transfected with the recombinant plasmid pCDNA3.1-LMP2 carrying the LMP2 gene sequence by liposome method (Lipofectamine 2000 Reagent, Invitrogen). After transfection, they were cultured with RPMI 1640 medium containing 10% bovine serum at 37°C for 24 hours, and then replaced with selective medium containing 600 μg / ml G418 to continue culturing. Three weeks later, positive cells with stable resistance were obtained. After continuing to expand the culture, they were used as target cells. 4 weeks old BALB / c(H-2 d ) male mice were randomly divided into 2 groups, 6 in each group, and each animal was 5×10 10 The number of virus particles was injected intramuscularly with the recombinant adeno-associated virus of this name and the control virus, respectively, to immunize animals. Booster immuniz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com