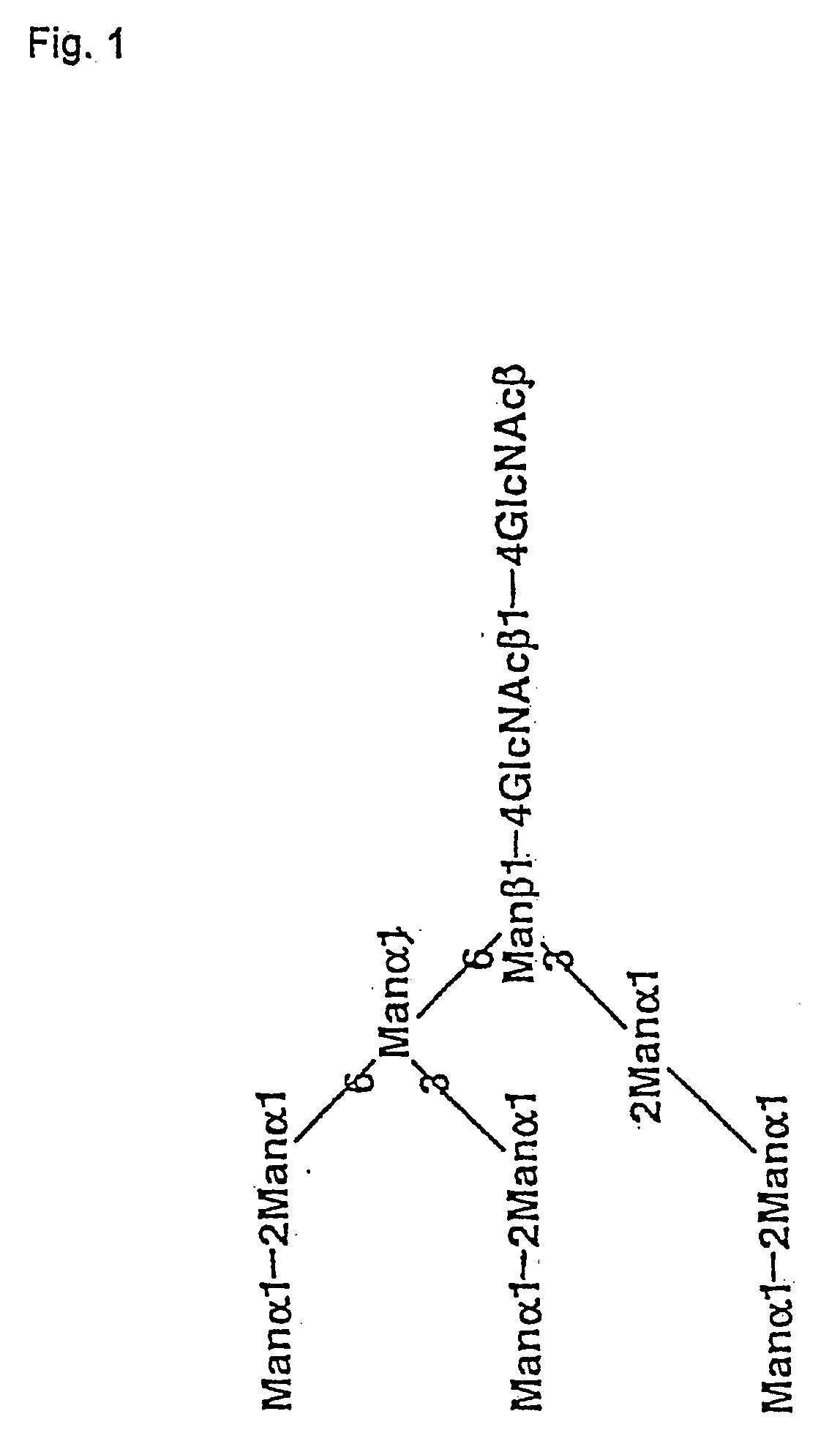
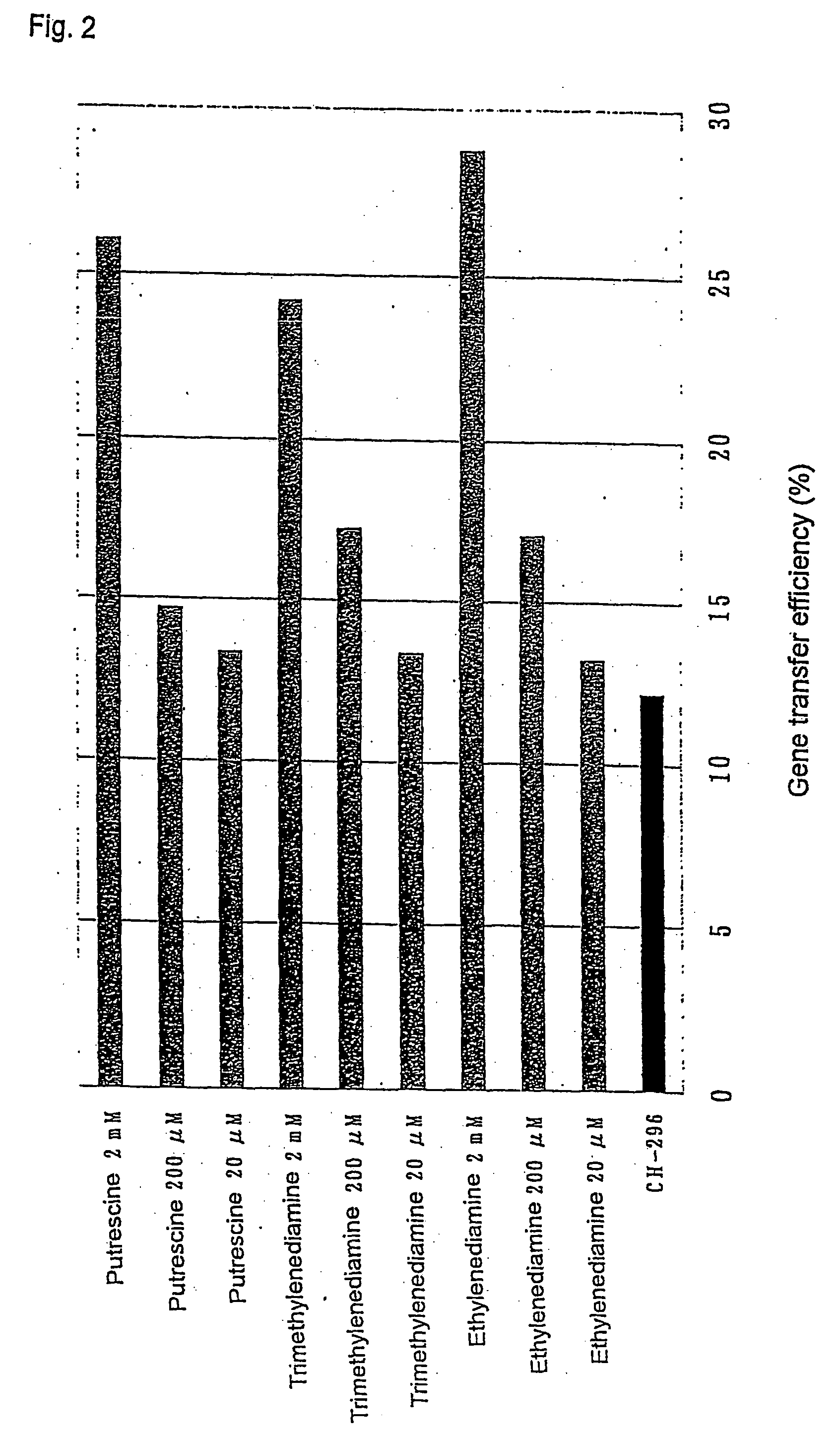
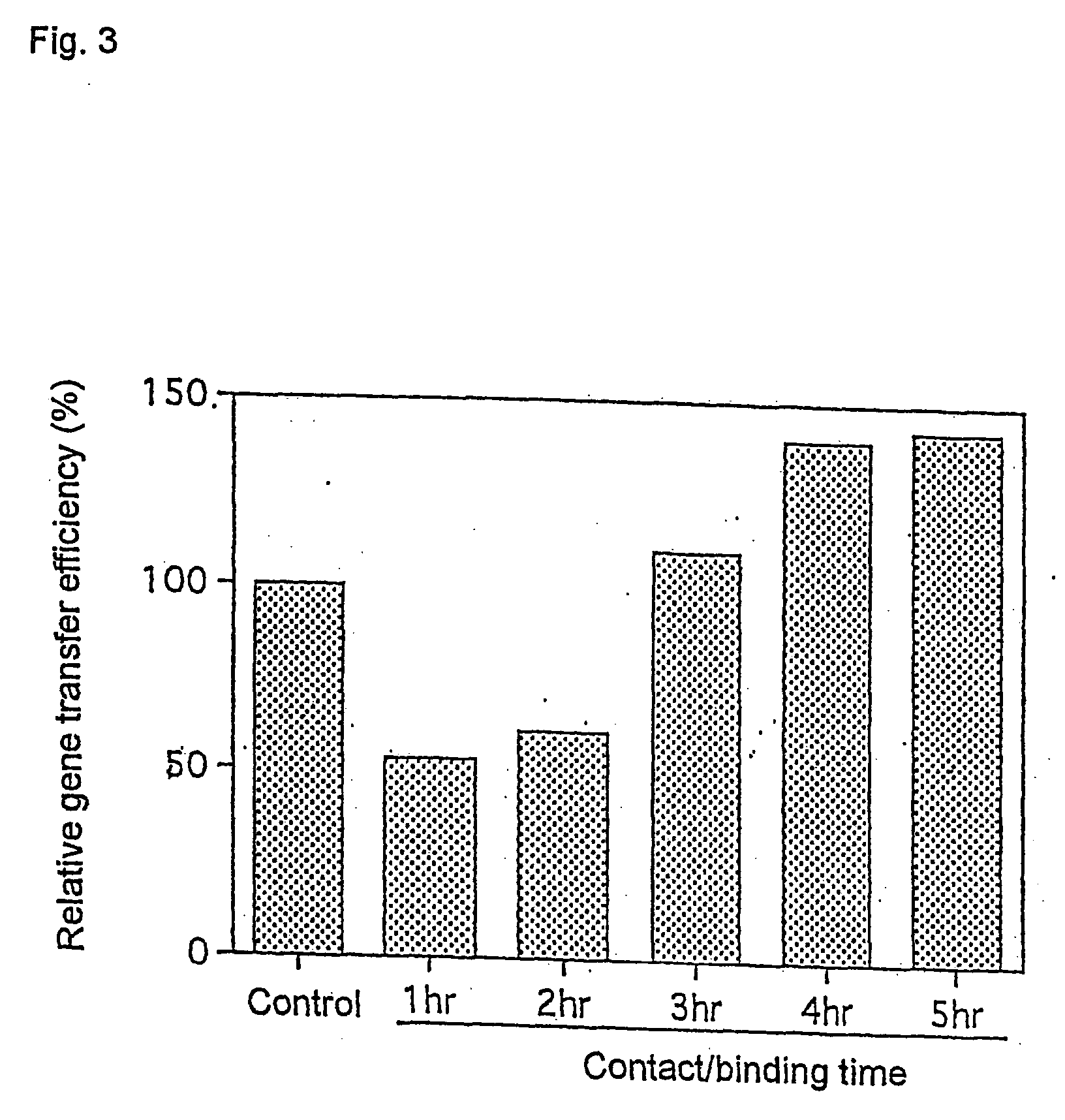
Gene transfer methods
a gene transfer and gene technology, applied in the field of gene transfer methods, can solve the problems of unsatisfactory practical clinical application of gene transfer efficiency using a retrovirus, contaminated retrovirus-producer cells, and high labor intensity of virus-producer cells, and achieve the effect of enhancing the gene transfer efficiency of laminin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0078] Preparation of Polypeptides derived from Fibronectin
[0079] A polypeptide derived from human fibronectin, H-271, was prepared from Escherichia coli HB101 / pHD101 (FERM BP-2264) containing a recombinant plasmid pHD101 which contains a DNA encoding the polypeptide according to the method as described in U.S. Pat. No. 5,198,423.
[0080] A polypeptide derived from human fibronectin, H-296, was prepared from Escherichia coli HB101 / pHD102 (FERM P-10721) containing a recombinant plasmid pHD102 which contains a DNA encoding the polypeptide according to the method as described in the above-mentioned publication.
[0081] A polypeptide CH-271 was prepared as follows.
[0082] Briefly, Escherichia coli HB101 / pCH101 (FERM BP-2799) was cultured according to the method as described in the above-mentioned publication. CH-271 was obtained from the culture.
[0083] A polypeptide CH-296 was prepared as follows.
[0084] Briefly, Escherichia coli HB101 / pCH102 (FERM BP-2800) was cultured according to the metho...
example 2
[0088] Construction of Retrovirus Vector and Preparation of Retrovirus Supernatant
[0089] A retrovirus plasmid, PM5neo vector, which contains a neomycin phosphotransferase gene [Exp. Hematol., 23:630-638 (1995)] was introduced into GP+E-86 cells (ATCC CRL-9642). The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Bio Whittaker) containing 10% fetal calf serum (FCS; Gibco), 50 units / ml of penicillin and 50 .mu.g / ml of streptomycin (both from Gibco). All of the DMEMs used in the procedure hereinbelow contained the above-mentioned antibiotics. A supernatant containing PM5neo virus was prepared by adding 4 ml of DMEM containing 10% FCS to a plate (a 10-cm gelatin-coated dish for cell culture, Iwaki Glass) in which the above-mentioned producer cells had been grown to semi-confluence, culturing overnight and then collecting the supernatant. The thus collected culture supernatant was filtrated through a 0.45-micron filter (Millipore) to prepare a virus supernatant stock, wh...
example 3
[0093] Preparation of Functional Substance having Activity of Binding to Retrovirus and Measurement of Activity thereof
[0094] 50 .mu.l of 80 .mu.g / ml solution of H-271, H-296, C-274, CH-271, CH-296, ColV, human basic fibroblast growth factor (bFGF; Progen), tenascin (Gibco) or epidermal growth factor (EGF; Takara Shuzo), or 50 .mu.l of 2% bovine serum albumin (BSA, Sigma) was added to each well of a 96-well non-treated microplate for cell culture (Falcon). The plate was allowed to stand at 4 C. overnight and then washed twice with phosphate buffered saline (PBS; Roman Kogyo). Alternatively, after the plate was treated as described above, 0.1 ml of 4 mg / ml solution of 1-ethyl-3-dimethylaminopropylcarbodiimide hydrochloride (Sigma) in sterile pure water was dispensed to each well. The reaction was allowed to proceed at 37 C. for 2 hours. The plate was washed extensively with pure water to prepare a carbodiimide-treated plate. These plates were stored at 4 C. until viral infection expe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com