Kit for detecting an EB viral capsid antigen IgA antibody
An EB virus and antibody detection technology, applied in biological tests, measuring devices, material inspection products, etc., can solve the problems of low degree of automation, high subjectivity, and large differences in result reporting, and achieve automatic detection, avoiding specificity and sensitivity. Affected effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of Epstein-Barr virus capsid antigen IgA antibody detection kit
[0022] 1. Preparation of VCA antigen-coupled magnetic particle suspension
[0023] 1.1 Magnetic particle washing
[0024] Take 200ul of magnetic particles containing carboxyl groups on the surface and put them in a glass bottle, use a magnet to adsorb the magnetic particles to the bottom of the glass bottle, remove the supernatant; add 2ml of 0.02M PBS (pH 8.0), repeat the above operation 3 times.
[0025] 1.2 Activation of magnetic particles
[0026] Dissolve EDC and NHS in 0.1M MES (pH 5.0) buffer solution at a concentration of 10-70mg / ml, then add 1ml of each to the magnetic particles; shake gently at room temperature for 30-60 minutes; The particles are adsorbed at the bottom, remove the supernatant; then add 2ml 0.1M MES (pH 5.0) buffer to resuspend the magnetic particles; repeat the above operation twice.
[0027] 2. Preparation of anti-human IgA monoclonal antibody enzyme c...
Embodiment 2
[0036] Embodiment 2 The detection method of kit of the present invention
[0037] The kit prepared in Example 1 was used in conjunction with the automatic chemiluminescence instrument AutoLumo A2000 or AutoLumo A2000 Plus produced by Zhengzhou Antu Bioengineering Co., Ltd. to detect respiratory syncytial virus IgM antibody:
[0038] Add 3 wells of positive control (for determining the Cutoff value) and 2 wells of negative control in sequence in the reaction container (hereinafter referred to as "well"), 100 µL / well; add 10 µL sample and 100 µL sample diluent to each well of the remaining wells;
[0039]Add 20 µL of magnetic particle suspension to each well; mix well, incubate at 37°C for 15 minutes; then wash with washing solution 5 times;
[0040] Add 50 µL of enzyme conjugate and 50 µL of antigen solution to each well; mix well and incubate at 37°C for 17 minutes; wash with cleaning solution 5 times;
[0041] Finally, add 50 µL each of Substrate A and Substrate B to each we...
Embodiment 3
[0045] Embodiment 3 Performance evaluation of the kit of the present invention
[0046] 1. Repeatability
[0047] Repeatability refers to the repeated measurement of the same sample, and the closeness of each measurement result to the mean value, expressed in the coefficient of variation CV.
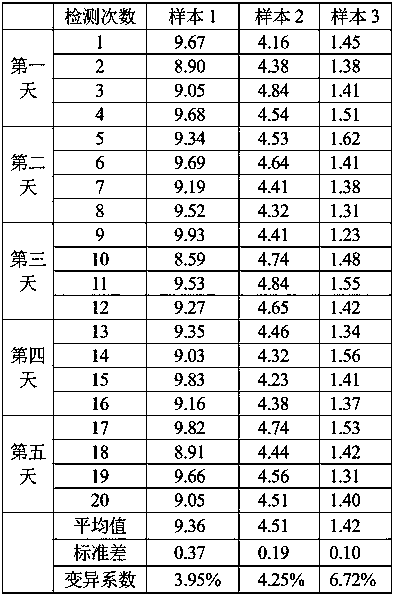
[0048] Select 3 samples with different concentrations, test 4 repetitions every day, and continuously test for 5 days, the coefficient of variation CV<8%, the results are shown in Table 1:
[0049] Table 1
[0050]
[0051] From the results in Table 1, it can be seen that the coefficient of variation of sample 1 is 3.95%, the coefficient of variation of sample 2 is 4.25%, and the coefficient of variation of sample 3 is 6.72%, all of which are less than 10% required by the industry, which proves that its repeatability is good.
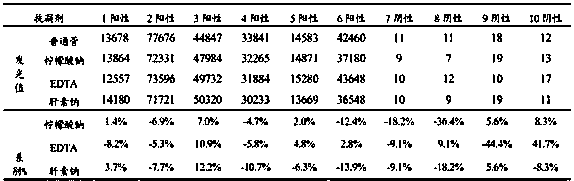
[0052] 2. Specificity
[0053] Detect hepatitis B, hepatitis C, syphilis, HIV, EB-EA, EB-NA1, HSV-1, HSV-2, CMV, VCA-IgG, VCA-IgM and HAMA positive samples,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com