Anti EB virus resulted tumour polypeptide, and its use and preparing method
An Epstein-Barr virus, anti-tumor technology, applied in anti-tumor drugs, anti-viral agents, botanical equipment and methods, etc., can solve the problem of not many specific markers on the cell surface, and achieve the effect of specific targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Construction of plasmids expressing anti-tumor polypeptides and preparation of recombinant anti-tumor polypeptides
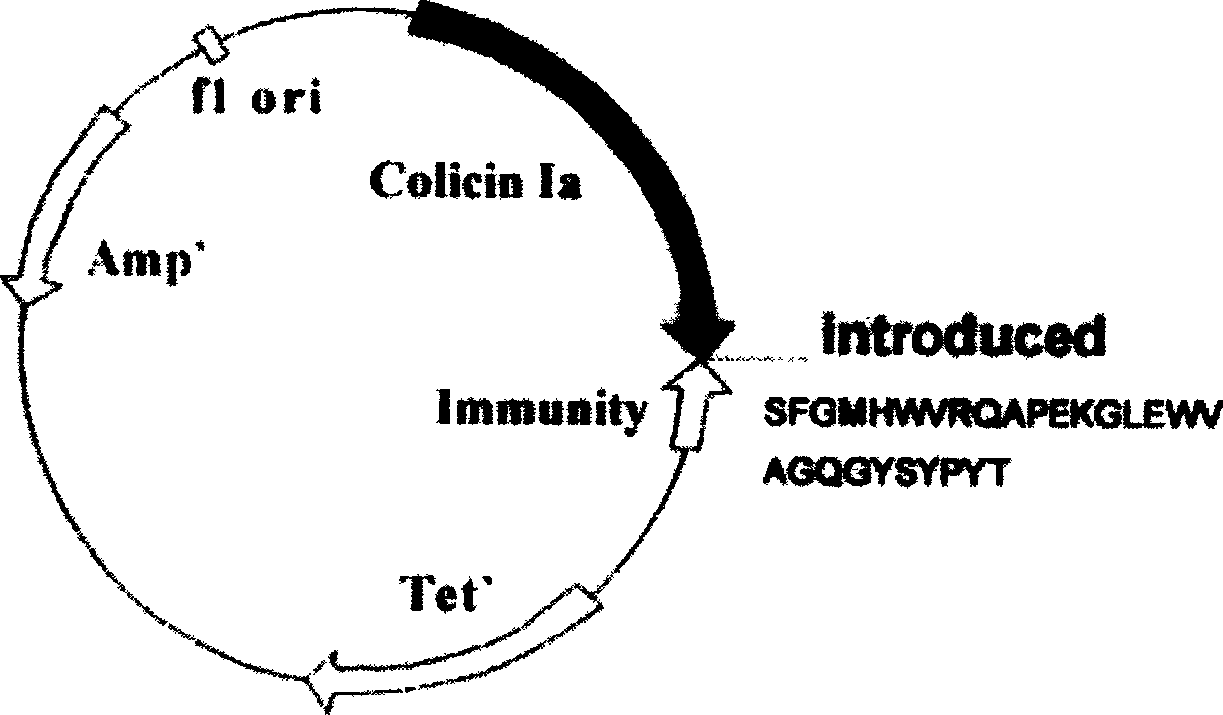
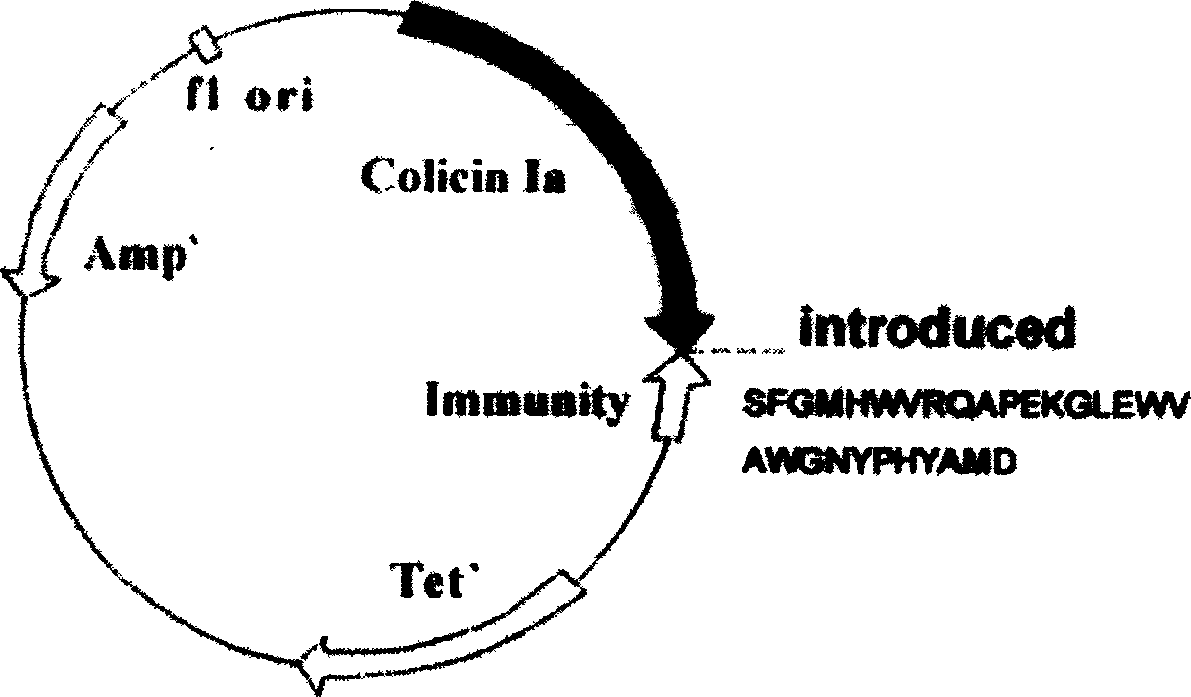
[0020] The original plasmid is pSELECT loaded with colistin Ia and Immunity protein genes TM -1 commercial plasmid (plasmid size 8.3kb, donated by UCSF Dr.P.Gosh), double-stranded oligonucleotide point mutation technology (QuickChange TM Kit, Strategene Company) inserts the gene (the nucleotide sequence described in SEQ ID NO.2 and SEQ ID NO.4 in the sequence listing) encoding the induced polypeptide into the I626 site of the colicin Ia gene, and has prepared the anti- Two recombinant plasmids pCHCEB1 (such as figure 1 shown), pCHCEB2 (as shown figure 2 shown). The recombinant plasmid was transfected into E. coli TG1 engineering bacteria (AECOM, donated by Dr. K. Jakes) to prepare the polypeptide, and the anti-tumor polypeptide described in SEQ ID NO.7 and SEQ ID NO.9 in the sequence listing was obtained. Wherein, the anti-tumor polypeptide ...
Embodiment 2
[0076] Embodiment 2: Recombinant anti-tumor polypeptide is used for external killing of human malignant lymphosarcoma (Burkitt's lymphoma) caused by Epstein-Barr virus
[0077] Epstein-Barr virus positive and negative cell lines: American ATCC standard cell lines.
[0078] Cell culture: Take out 0.1ml of revived cultured Raji cell suspension, slowly add it into a petri dish containing 3ml 1640 liquid medium (plus 10% serum) (dilution ratio is 1:30), mix well, put in 37°C CO 2cultured in an incubator. A Epstein-Barr virus-positive cell line was used in the experiment: ATCC CCL-86 (the standard human Burkitt's malignant lymphosarcoma cells used in cell culture laboratories all over the world, Raji cells, isolated from a 12-year-old African male child in 1963). The test cells were divided into 4 groups. The first group was the blank group, which was added with recombinant anti-tumor polypeptide blank preservation solution (10mMPB+0.2M NaCI phosphate buffer (pH7.4)). The second ...
Embodiment 3
[0080] Example 3: Multiple fluorescent staining observation of the in vitro killing effect of recombinant anti-tumor polypeptide on Burkitt's human malignant lymphosarcoma cells and other tumor cells and normal cells caused by Epstein-Barr virus
[0081] Cell culture conditions are the same as in Example 2. A total of seven cell lines were used in the experiment, Epstein-Barr virus-positive cell lines: ATCC CCL-86 (Raji cells, Burkitt's malignant lymphosarcoma cells); ATCC CRL-2230, 46-year-old male AIDS patient (AIDS) malignant lymphosarcoma cell lines, Epstein-Barr virus and Kaposi sarcoma virus (HHV8) positive; Epstein-Barr virus negative cell lines: ATCC CRL-1648 (CA-46, cells isolated from ascites of American Burkitt's malignant lymphosarcoma patients); ATCCHepG2 human hepatoma cells; SMMC-7721 human liver cancer Cells, 3T3 mouse embryonic fibroblasts; ECV-304 human umbilical vein endothelial cells (the latter three cells are all from the Cell Center of Peking Union Medic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com