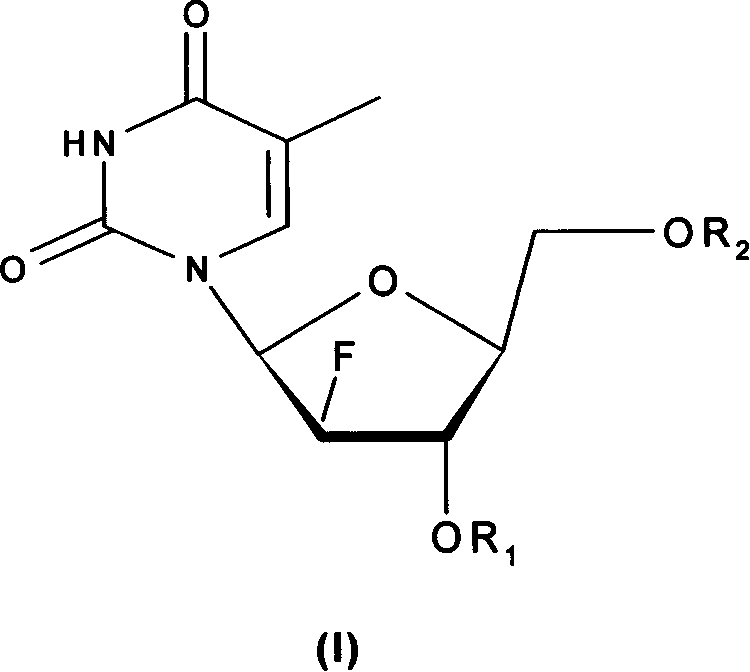
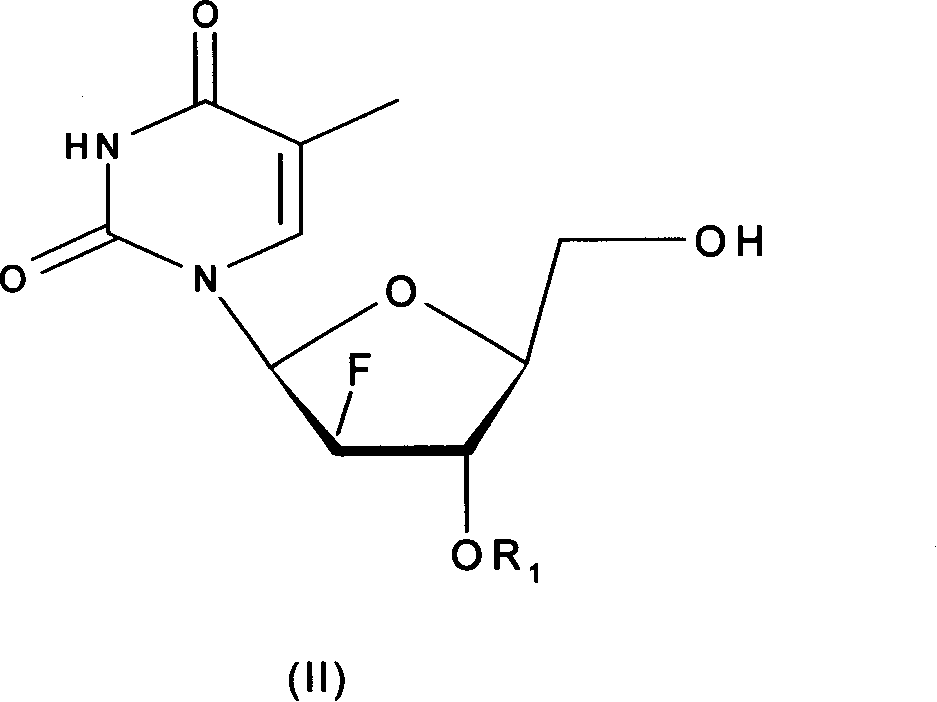
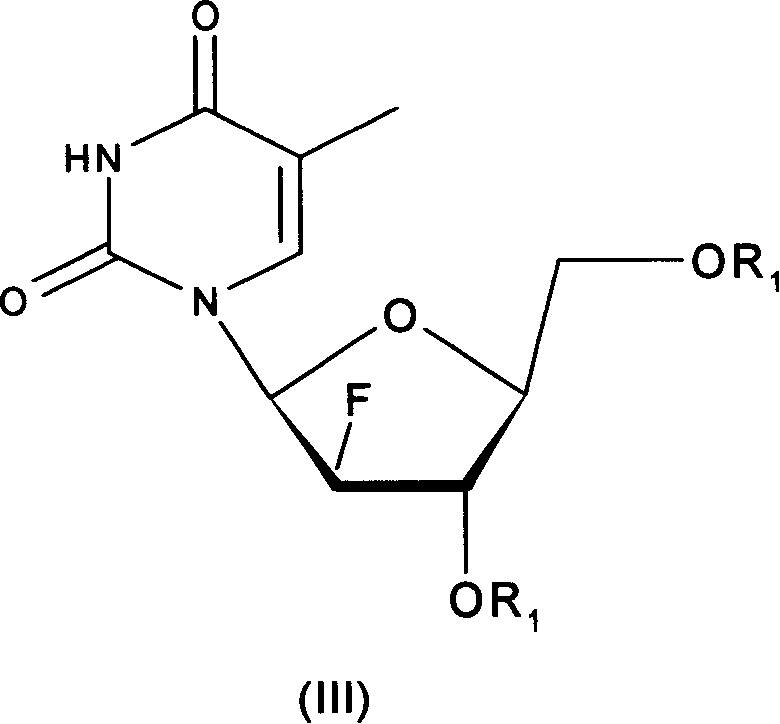
L-nucleoside prodrug
A technology of antiviral drugs and compounds, applied to the pharmaceutical composition of prodrugs and its preparation, application in the preparation of antiviral drugs, the field of preparation of prodrugs, can solve the problem of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Preparation of 1-O-methyl-L-ribofuranose
[0105]
[0106] HCl gas was passed into methanol (250ml) at 0°C until the solution was saturated, then L-ribose (50g) was dissolved therein, and HCl gas was continued to react for 3hr.
[0107] The reaction solution was neutralized with solid sodium carbonate to pH = 7, filtered, and the reaction solution was evaporated to dryness under reduced pressure to obtain 60 g of 1-O-methyl-L-ribofuranose in the form of yellow syrup, which was directly used in the next reaction.
Embodiment 2
[0108] Example 2 Preparation of 1-O-methyl-2,3,5-tri-O-benzoyl-L-ribofuranose
[0109]
[0110] Dissolve 60 grams of 1-O-methyl-L-ribofuranose in pyridine (800ml), stir at 0°C, add benzoyl chloride (200ml) dropwise, the reaction solution changes from light yellow to pink, and a large amount of solids appear (pyridine hydrochloride), then return to room temperature and stir the reaction for 17hr.
[0111] After the above reaction solution was suction filtered, the solid was discarded and the filtrate was evaporated to dryness under reduced pressure, and the residue was added with CH 2 Cl 2 (500ml) was dissolved, followed by 1% HCl solution (500ml×3), aqueous solution (500ml×3), KHCO 3 solution (500ml×2) for washing. Activated carbon (50g) was added to the organic layer, heated to reflux for 0.5hr decolorization, and the liquid was cooled to room temperature with anhydrous Na 2 SO 4 Remove water, then filter through a bed of silica gel and sand to remove color. Finally,...
Embodiment 3
[0112] Example 3 Preparation of 1-O-acetyl-2,3,5-tri-O-benzoyl-β-L-ribofuranose
[0113]
[0114] The 1-O-methyl-2,3,5-tri-O-benzoyl-L-ribofuranose obtained in the previous reaction was mixed with 72mlAcOH and 167mlAcOH 2 O was dissolved in a 1000ml round bottom flask, stirred at 0°C and 24ml conc-H was added dropwise 2 SO 4 , the solution changed from orange-red to brown-black. After the dropwise addition, the reaction solution was left to recover to room temperature, and then placed in the refrigerator overnight.
[0115] The reaction solution was poured into 350ml of ice water, stirred evenly and left to stand, the liquid was layered, 1000ml of ethyl acetate was added, and the solution was mixed with aqueous solution (500ml×3), KHCO 3 solution (500ml×2), brine (500ml×2) for extraction. Separate the organic layer with anhydrous NaSO 4 After removing water, add activated carbon (50 g), heat to reflux for 0.5 hr, cool the liquid to room temperature, and filter through a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com