Chimeric particle containing dominant epitope peptide of EB virus membrane surface glycoprotein gp350 and coding gene and application of chimeric particle
An Epstein-Barr virus and dominant epitope technology, applied in the field of genetic engineering, can solve the problems such as the lack of good treatment methods for cancer and the lack of Epstein-Barr virus preventive vaccines, and achieve the effect of improving immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Construction of recombinant expression vector and expression of fusion protein
[0033] 1. Experimental materials
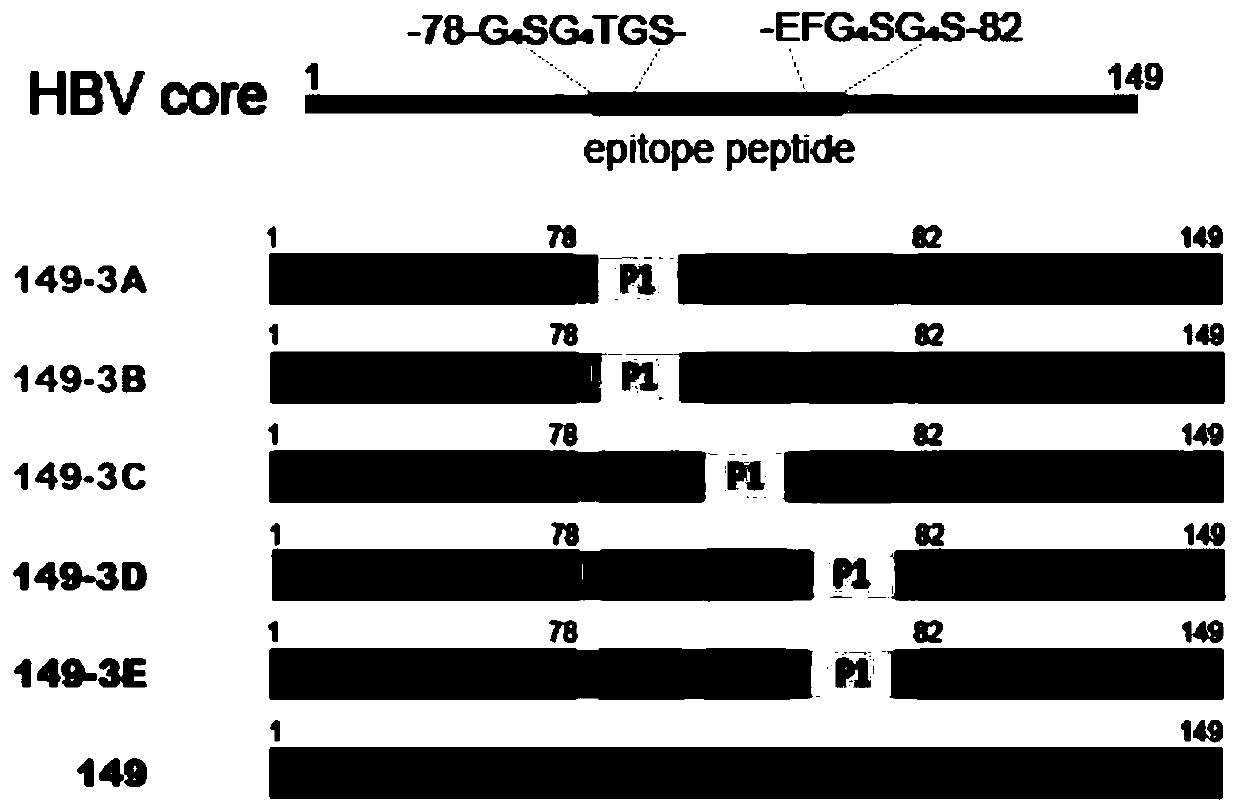
[0034] (1) The present invention selects the hepatitis B core protein HBc149 as the granulated display carrier, transforms the carrier, replaces the 79-81 amino acids of HBc149 with "GGGGSGGGGTGSEFGGGGSGGGGS" and introduces BamH I and EcoR I double enzyme cleavage sites (GS / EF) , to facilitate the insertion of subsequent epitopes into recombinant expression vectors by enzyme-cut ligation.
[0035] (2) Reagents and consumables: All reagents and consumables are commercially available.
[0036] (3) Host cell: BL21DE3.
[0037] 2. Steps
[0038] (1) In order to fully investigate the ability of the dominant epitope to induce humoral immune response, the selected gp350 dominant epitope peptides were randomly combined and inserted into the granulated vector to construct an expression vector;
[0039] (2) Transform the above-mentioned vector into ...
Embodiment 2
[0054] Example 2 Immunoreactivity Verification of Recombinant Proteins
[0055] 1. Experimental materials
[0056] (1) Reagents and consumables: All reagents and consumables are commercially available.
[0057] (2) Antibody: HBc149-specific antibody 11H10 was a kind gift from Professor Xia Ningshao of Xiamen University; anti-gp350ECD123 multiple antiserum was prepared by immunizing mice in our laboratory.
[0058] 2. Experimental steps
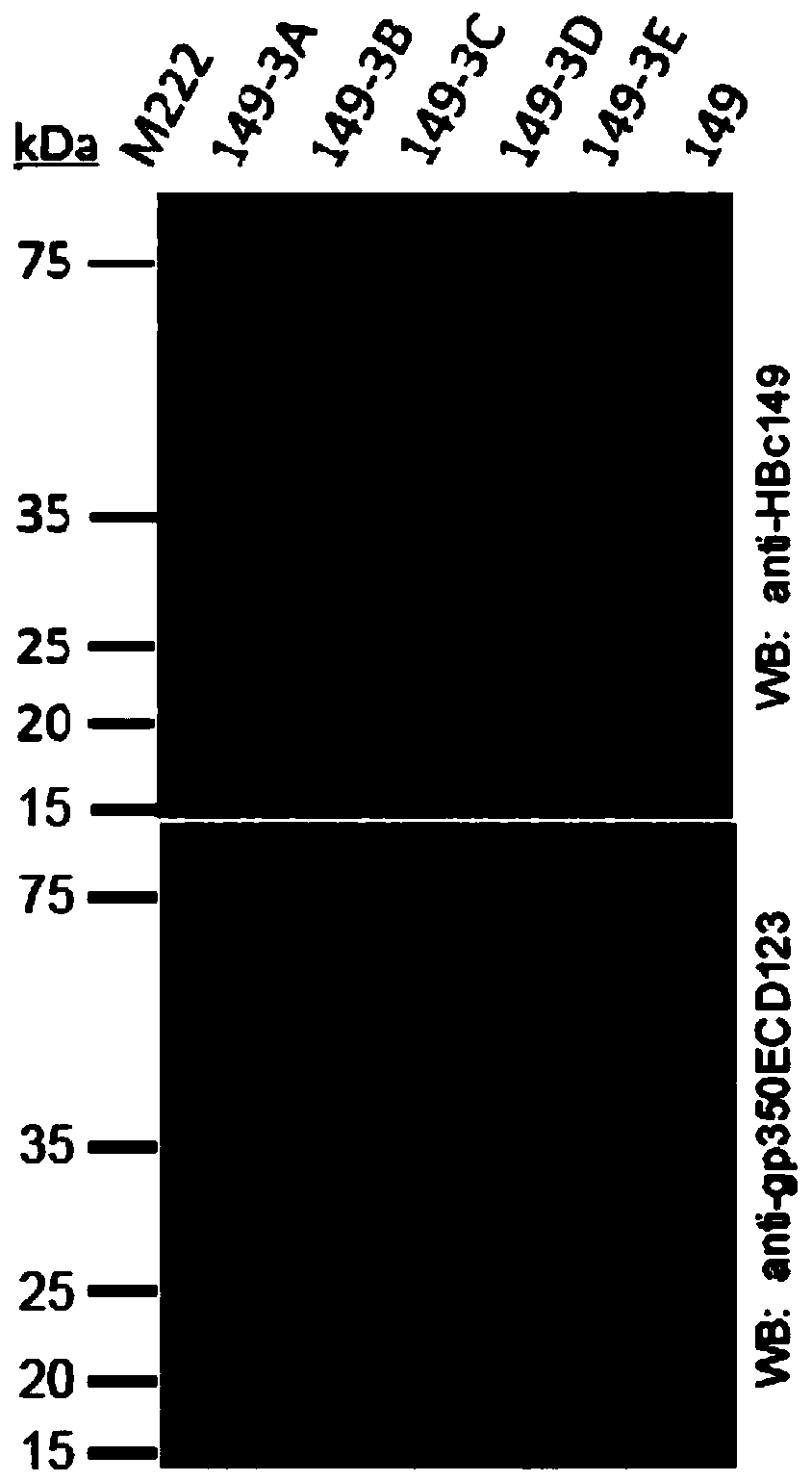
[0059] (1) SDS-PAGE electrophoresis for the recombinant protein;
[0060] (2) After the electrophoresis is completed, the protein is transferred to the PVDF membrane;
[0061] (3) After the membrane transfer was completed, the PVDF membrane was blocked with 5% skimmed milk, and then the primary antibody and the secondary antibody labeled with horseradish peroxidase were incubated, and finally the substrate of horseradish peroxidase was used for color development.
[0062] 3. Experimental results
[0063] Such as image 3 As shown, the ex...
Embodiment 3
[0064] The granularity of embodiment 3 recombinant protein
[0065] 1. Experimental materials
[0066] (1) Reagents and consumables: All reagents and consumables are commercially available.
[0067] (2) Instruments: transmission electron microscope; Agilent HPLC analyzer; molecular sieve G5000PWxl.
[0068] 2. Experimental steps
[0069] (1) Staining the purified protein with tungsten phosphate;
[0070] (2) Transmission electron microscope detection after staining.
[0071] (3) After the purified protein is diluted to 0.5 mg / mL, the molecular sieve detection is carried out by using a high performance liquid chromatography analyzer.
[0072] 3. Experimental results
[0073] Such as Figure 4 As shown in A, the transmission electron microscopy test results show that all recombinant proteins have formed uniform particles; in addition Figure 4 B was detected by molecular sieves, and the results also showed that compared with the control protein wild-type HBc149, all recom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com