Polypeptide-modified liposome, mRNA delivery system and dendritic cell vaccine
A technology of liposomes and auxiliary lipids, applied in the field of biomedicine, can solve problems such as the need to improve transfection efficiency, and achieve the effects of enhancing anti-tumor effect, strong immune function, and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] For example, in an example of the present invention, liposomes were further made using DP7-C, DOTAP and cholesterol as raw materials. And provide a kind of typical preparation method based on film dispersion method, the step of this method is:
[0099] a, weigh DOTAP and cholesterol and dissolve in the solvent, and remove the solvent by rotary evaporation under reduced pressure;
[0100] b. The aqueous solution of the hydrophobically modified cationic polypeptide is added to the system for hydration and membrane removal to obtain a liposome suspension;
[0101] c. ultrasonically processing the liposome suspension obtained in b to obtain liposomes.
[0102] The solvent used in step a is generally an organic solvent, and commonly used ones include chloroform, ethanol, methanol, dichloromethane, ethyl acetate and the like.
[0103] During sonication, it is generally necessary to control the temperature of the system not to be too high, preferably below 25°C. For example,...
Embodiment 1
[0153] Embodiment 1, preparation and characterization of liposomes modified by DP7-C
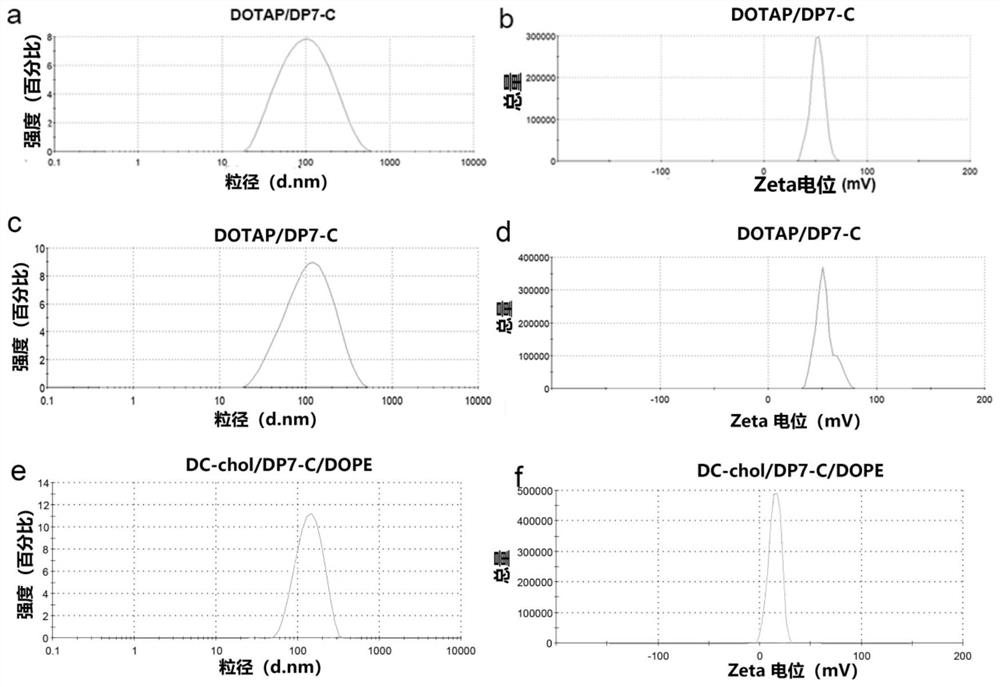
[0154] In this example, we adopted different methods (film dispersion method and ethanol injection method) to prepare DP7-C modified DOTAP / cholesterol cationic liposomes, and prepared DP7-C modified DC-chol / DOPE cations by film dispersion method Liposomes.
[0155] 1. Preparation and characterization of DP7-C modified DOTAP liposomes
[0156] 1) Prepare DOTAP liposomes modified by DP7-C by film dispersion method
[0157] Weigh 20mg DOTAP and 20mg cholesterol, dissolve them in 4ml chloroform, and add them to a 100ml eggplant-shaped bottle. The organic solvent was removed by rotary evaporation in a water bath at 37° C. under reduced pressure, and the rotation speed was set at 50 rpm / min. Add 10ml of 300μg / ml DP7-C aqueous solution into the eggplant-shaped bottle for hydration and film removal, set the temperature at 60°C, and the rotation speed at 75rpm / min, and continue hydration for 30min...
Embodiment 2
[0167] Preparation and characterization of the DOTAP liposome complex mRNA of embodiment two, DP7-C modification
[0168] 1. The method and optimal ratio of DOTAP liposomes modified by DP7-C and mRNA
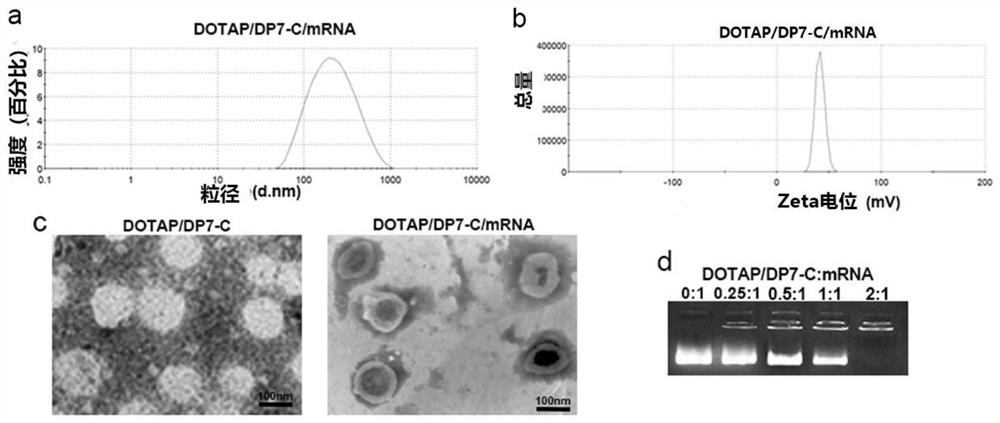
[0169] The DP7-C modified DOTAP liposome prepared in Example 1 was diluted in an aqueous solution to the use concentration. Add mRNA according to the ratio of liposome:mRNA=2:1, mix gently, and incubate at room temperature for 10 minutes to obtain DP7-C modified DOTAP liposomes. Gel retardation electrophoresis detected the combination of DP7-C modified liposomes and mRNAs of different mass ratios (mRNA encoding neoantigens shown in SEQ ID No.4, the same below), and DP7-C modified liposomes The mass ratios of plastid and mRNA were 0:1, 0.25:1, 0.5:1, 1:1, 2:1, respectively.
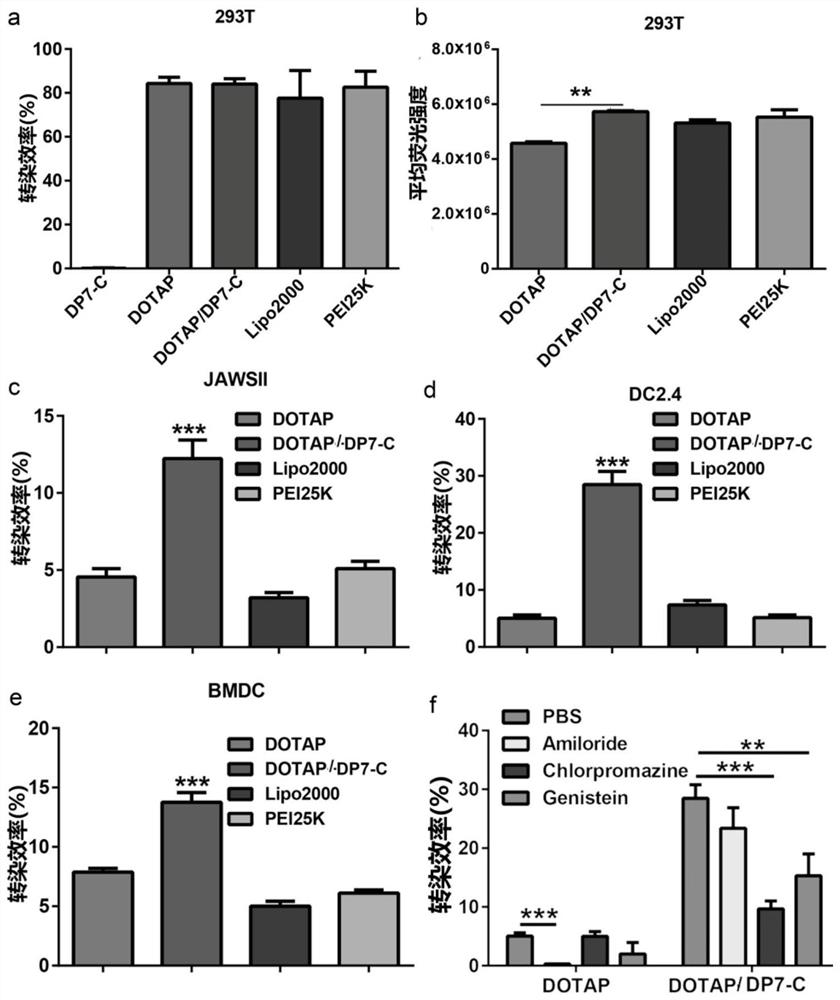
[0170] The experimental results show that: the particle diameter of the DOTAP liposome composite mRNA modified by DP7-C is 130.45 ± 9.32nm, and the zeta potential is 34.67 ± 7.45mV ( figure 2 a-b). Tran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com