Tumor composite antigen, dendritic cell multivalent vaccine and application of dendritic cell multivalent vaccine
A technology of dendritic cells and composite antigens, applied in the field of biomedicine, can solve the problems of lack of anti-tumor immune response, tumor killing, and destruction of the immune system, so as to achieve good clinical application prospects, inhibit the evolution of cancer cells, and enhance the immune system Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
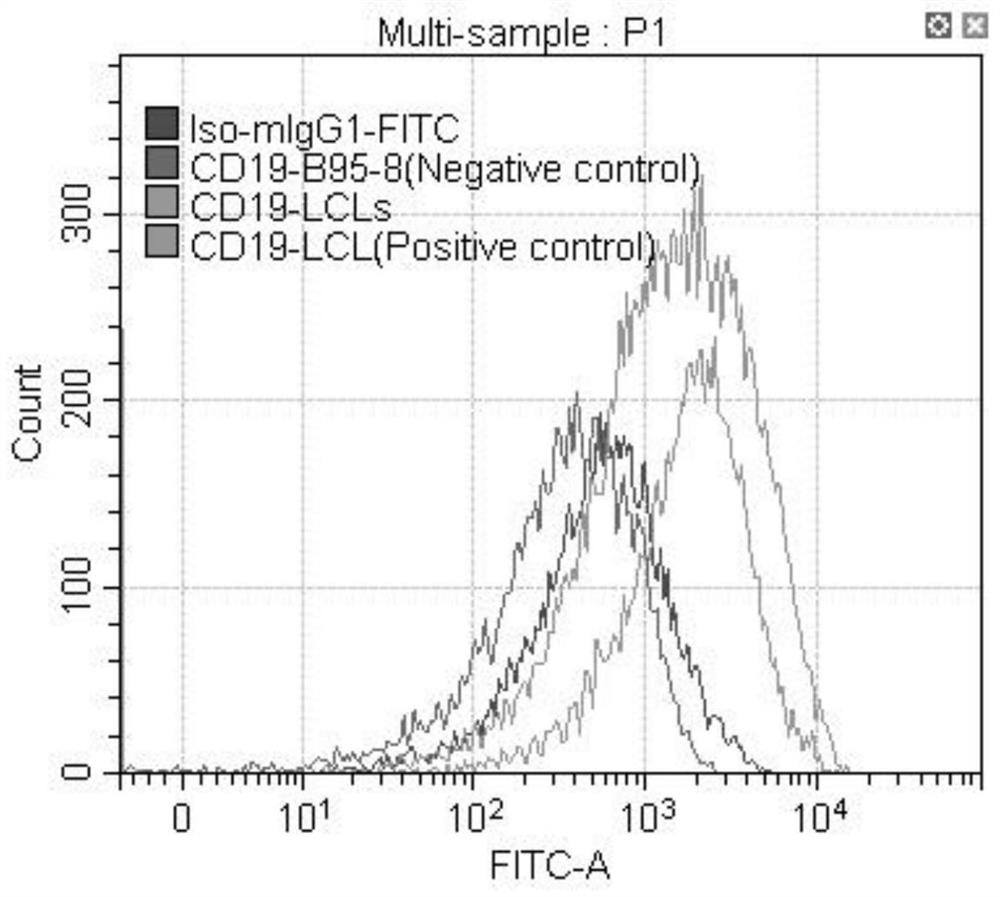
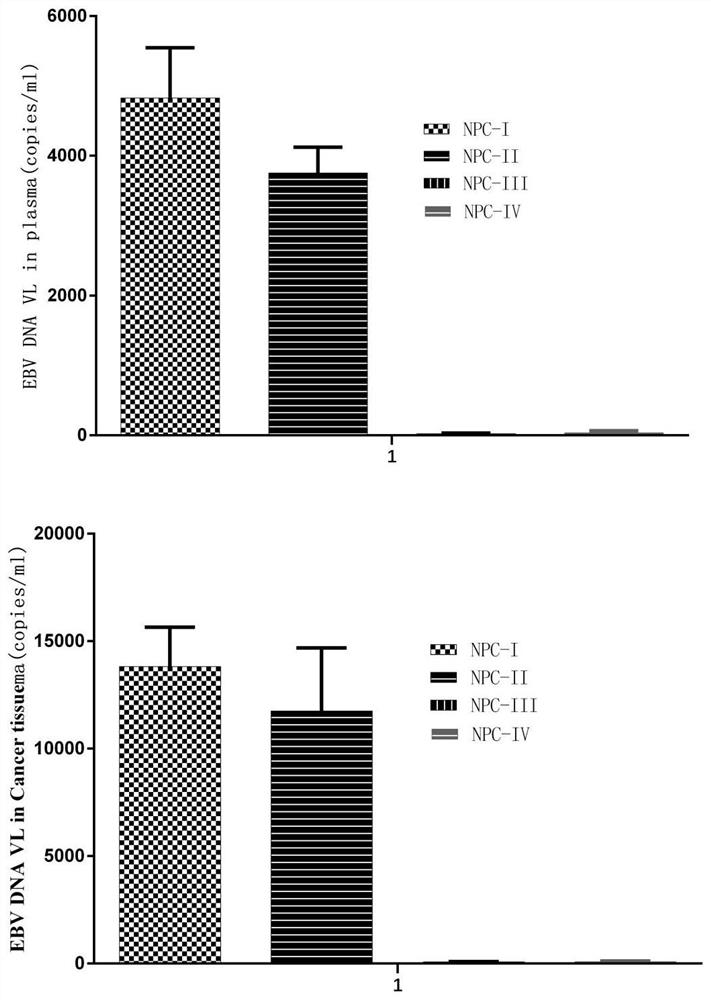
[0059] Example 1: Immunological research on the treatment of nasopharyngeal carcinoma by dendritic cell multivalent vaccine
[0060] The tumor cells of two EBV-positive nasopharyngeal carcinoma patients (marked as I, II) and the tumor cells of two EBV-negative nasopharyngeal carcinoma patients (marked as III, IV) were selected in this embodiment (obtained informed consent from the patients). ).
[0061] 1. Isolation of peripheral blood mononuclear cells from human venous blood
[0062]This embodiment is based on the difference in the density of each cell component in peripheral blood (peripheral blood mainly contains cells such as platelets, mononuclear cells, granulocytes, and red blood cells: the density of platelets is 1.030-1.035kg / m 3 , the mononuclear cell density is 1.075~1.090kg / m 3 , the granulocyte density is 1.092kg / m 3 , red blood cell density is 1.093kg / m 3 ), added in peripheral blood samples Paque Plus (GE Healthcare) solution (density 1.075~1.089kg / m 3 )...
Embodiment 2
[0151] Example 2: Study on Immunological Effects of Dendritic Cell Multivalent Vaccine against EBV-positive Gastric Cancer
[0152] Two EBV-positive gastric cancer patients (A, B) and two EBV-negative gastric cancer patients (C, D) selected in this example have obtained informed consent from the patients.
[0153] 1. Isolation of peripheral blood mononuclear cells from human venous blood
[0154] This embodiment is based on the difference in the density of each cell component in peripheral blood (peripheral blood mainly contains cells such as platelets, mononuclear cells, granulocytes, and red blood cells: the density of platelets is 1.030-1.035kg / m 3 , the mononuclear cell density is 1.075~1.090kg / m 3 , the granulocyte density is 1.092kg / m 3 , red blood cell density is 1.093kg / m 3 ), added in peripheral blood samples Paque Plus (GE Healthcare) solution (density 1.075~1.089kg / m 3 ), performing density gradient centrifugation to stratify different cell components, and can...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com