DC vaccine for novel corona viruses, and preparation method and application of DC vaccine
A coronavirus and vaccine technology, applied in the field of biomedicine, can solve problems such as the lack of DC vaccines, and achieve the effects of improving the killing effect, quick start, and shortening the time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the construction of S protein antigen expression vector
[0034] The nucleic acid sequence of each module of the S protein antigen plasmid construction is as follows:
[0035] (1) Leader CD8 (SEQ ID NO.1)
[0036] (2) 2019nCOV-S (SEQ ID NO.2)
[0037] (3) T2A (SEQ ID NO.3)
[0038] (4) CCL19 (SEQ ID NO.4)
[0039] Beijing Biomed Biotechnology Co., Ltd. was entrusted to sequentially synthesize artificial nucleic acid sequences according to the fusion genes CD8-2019nCOV-S (SEQ ID NO.5) and CD8-2019nCOV-S-T2A-CCL19 (SEQ ID NO.6), and insert them into The adeno-associated virus vector pAAV-IRES-hrGFP was transformed into E.coli (TOP10). After sequencing and identification, the plasmid was extracted and purified using the Qiagen plasmid purification kit to obtain recombinant adeno-associated virus expression vectors pAAV-2019nCOV-S and pAAV - 2019nCOV-S-CCL19 with a plasmid concentration higher than 200ng / ul.
Embodiment 2
[0040] Embodiment 2, packaging and titer determination of adeno-associated virus
[0041] ① Recovery and passage of HEK293 cells
[0042] Take out the frozen HEK293 cells from the liquid nitrogen tank, quickly throw them into a 37°C water bath and shake them quickly, try to completely dissolve the cell solution within 1-2 minutes. Transfer the cell solution to a 50ml centrifuge tube, add 5 ml of fresh complete medium (containing 10% FBS), mix well and centrifuge at 1500 rpm for 5 min. Remove the supernatant, add 1 ml of fresh complete medium to resuspend the cell pellet, transfer it to a T75 bottle, and make up to 10ml of complete medium in each bottle. Place the culture flask steadily at 37°C, 5% CO 2 and cultured in an incubator with 95% relative humidity. The cell viability was observed the next day, and the medium was replaced. Afterwards, the growth of the cells was observed every day, and the cells were passaged when the cells covered 80%-90% of the bottom of the bot...
Embodiment 3
[0049] Embodiment 3, DC vaccine preparation
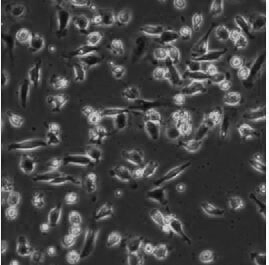
[0050] Collect 50ml of peripheral venous blood from a healthy donor, add 50ml of normal saline for dilution, first add 20ml of lymphocyte separation solution to a 50ml centrifuge tube, then slowly add 25ml of blood, and centrifuge at 900g for 25min. The liquid surface is divided into four layers, absorb the white blood cell layer in the middle, and add DC medium. 37°C, 5% CO 2 Incubate for 2 hours in an incubator to allow monocytes to adhere to the wall. Discard the culture supernatant, add DC medium (purchased from Dayou) containing recombinant human GM-CSF (500-1000U / ml) and recombinant human IL-4 (500U / ml) to the adherent cells, 37°C, 5 %CO 2 Culture in an incubator, change the medium every other day, and culture for 5 days to obtain imDC (such as figure 2 ).
[0051] Take out the prepared pAAV-2019nCOV-S and pAAV-2019nCOV-S-CCL19 viruses, and infect the above imDCs at MOI=5. After 8-12 hours of infection, wash the cells 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com