Efficient tumor antigen loaded DC vaccine and method for induced proliferation of tumor antigen specific CTL by same
A tumor antigen, specific technology, applied in the fields of medicine and biology, to achieve efficient and specific killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
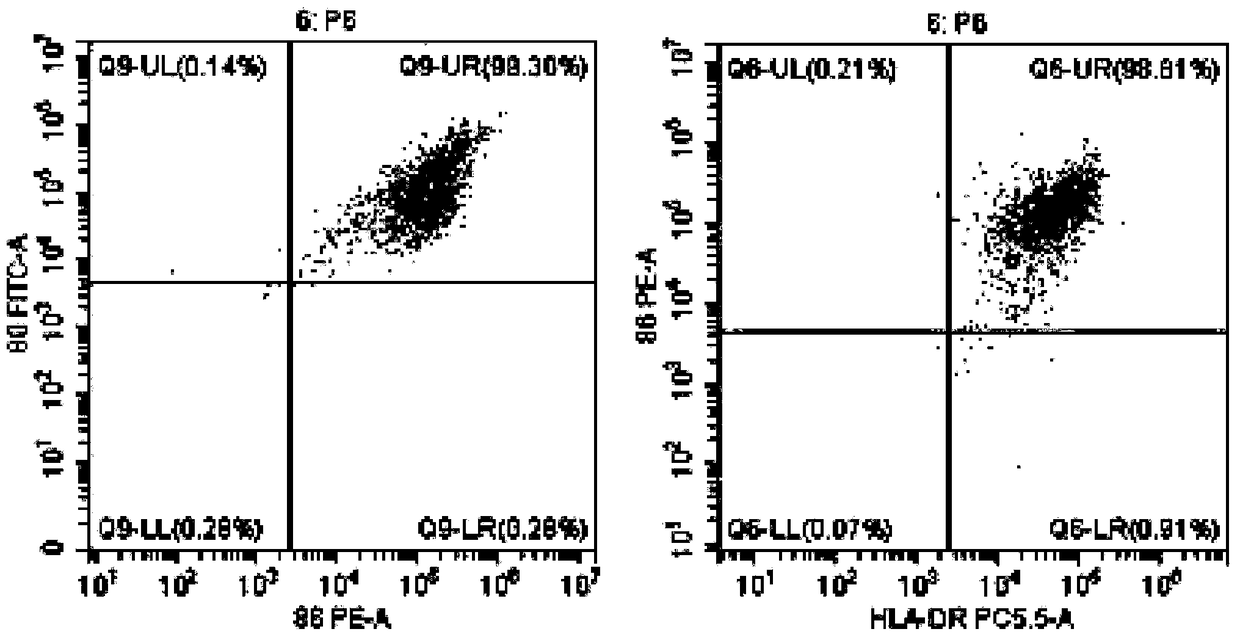
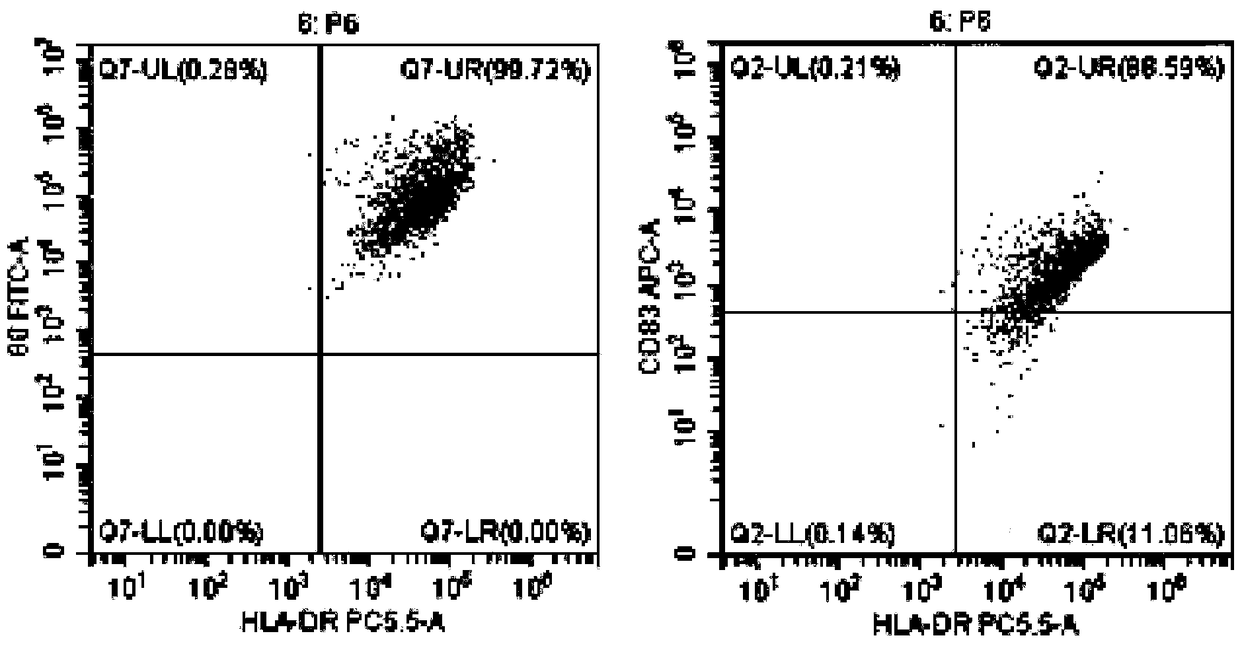
[0048] Example 1 Preparation of DC Vaccine Highly Efficiently Loaded with Tumor Antigens
[0049] 1. Tumor Cell Culture
[0050] Tumor cells come from the patient's own surgically resected tumor tissue or tumor tissue biopsy specimens, as well as tumor cell lines with the same pathological diagnostic characteristics as the patient. The cell line can be self-built in the laboratory, or can be purchased from China National Cell Collection Center or ATCC in the United States, and cultured in vitro according to the biological characteristics of the tumor cells. Usually use DMEM medium containing 10-15% fetal bovine serum at 37°C, 5% CO 2 Cultured in the incubator, subcultured and expanded.
[0051] 2. Induce cell apoptosis and express heat shock proteins
[0052] When the cells grow to the logarithmic phase and 80% of the bottom of the bottle is covered, the drug treatment is carried out, and arsenic trioxide (1-15umol / L can be added) is continued at 37 ° C, 5% CO 2 Incubate f...
Embodiment 2
[0071] Example 2 Preparation of DC Vaccine Highly Efficiently Loaded with Tumor Antigens
[0072] A method for preparing tumor antigens from tumor cells and exosomes in tumor cell culture fluid by separating tumor cells from the pleural and ascites of tumor patients, culturing and expanding them at one time.
[0073] 1. Collect 500-1000ml of pleural effusion or ascites from tumor patients under sterile conditions, centrifuge at 300g for 8 minutes, and discard the supernatant.
[0074] 2. Add physiological saline to 100ml to resuspend to make cell suspension.
[0075] 3. Double density centrifugation: from bottom to top: the first layer of 100% human lymphocyte separation liquid (density 1.077g / ml); the second layer of 75% lymphocyte separation liquid (density 1.077g / ml); the third layer cell suspension. Centrifugation: 700g, 20 minutes.
[0076] 4. Aspirate and collect the cells on the first interface and the second interface respectively, and wash with normal saline twice....
Embodiment 3
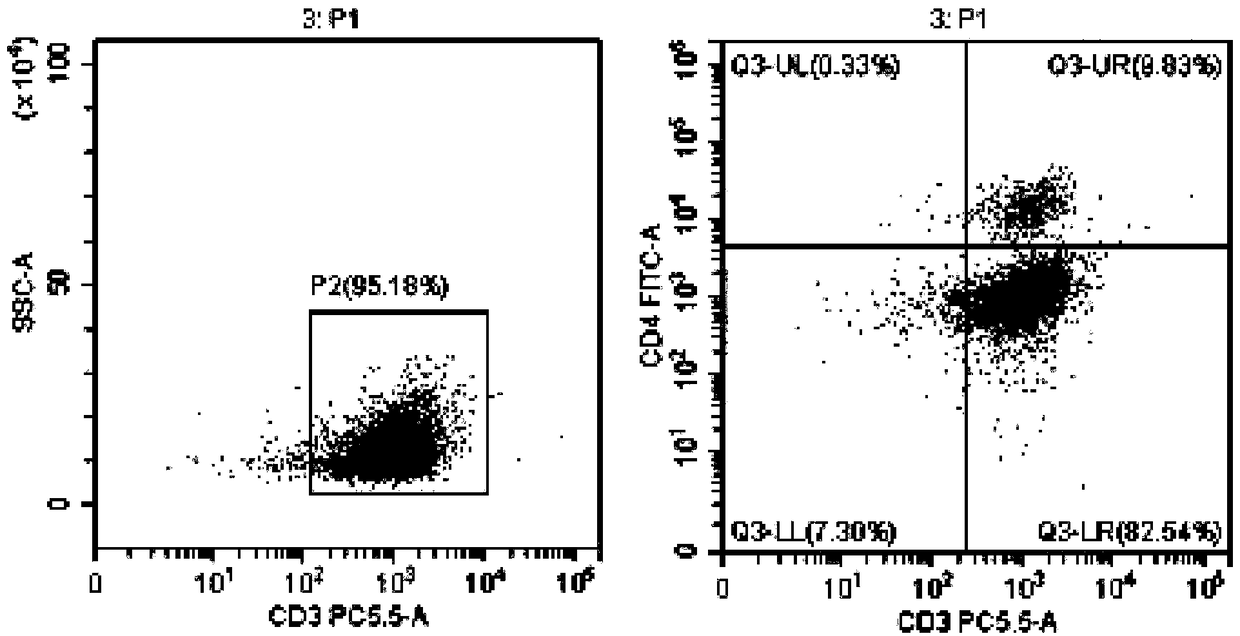
[0082] Example 3 Induction and expansion of tumor-specific CTL in vitro by tumor antigen-sensitized and activated DC
[0083] The DC vaccines prepared in Example 1 and Example 2 were selected respectively.
[0084] 1. On the day when the DC vaccine is harvested, draw 40-50 ml of heparin anticoagulated blood for the second time, and follow the operations of 1) and 2) in Step 6 of Example 1 to suck out the freshly separated non-adherent mononuclear cells, and use 5: The ratio of 1 was mixed with the prepared DC vaccine (DC vaccine prepared by referring to the method of Example 1 or Example 2), placed in a culture bottle containing serum-free CTL priming medium (BYN-PT331) overnight, and added anti-human CD3 monoclonal antibody, anti-human CD28 monoclonal antibody, rh-IL-2, at 37°C, 5% CO 2 cultured in an incubator. Afterwards, according to the growth and expansion of the cells, an appropriate amount of serum-free expansion medium (BYN-PT332) containing rh-IL-2 was added in an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com