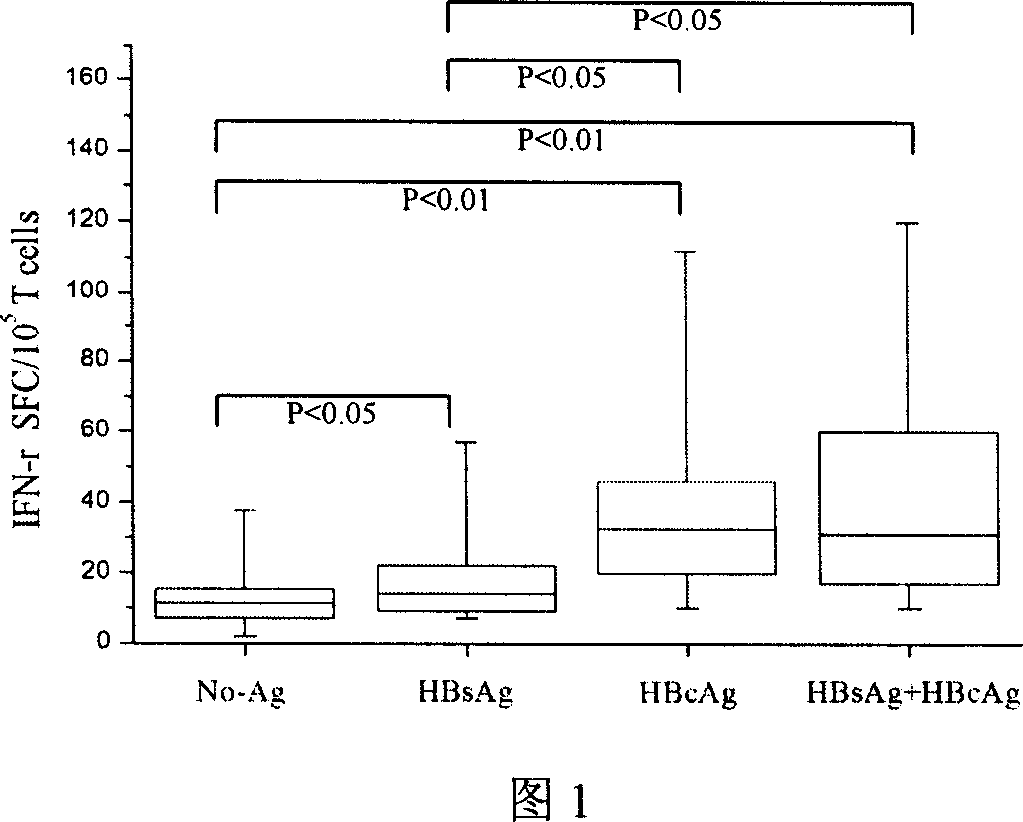
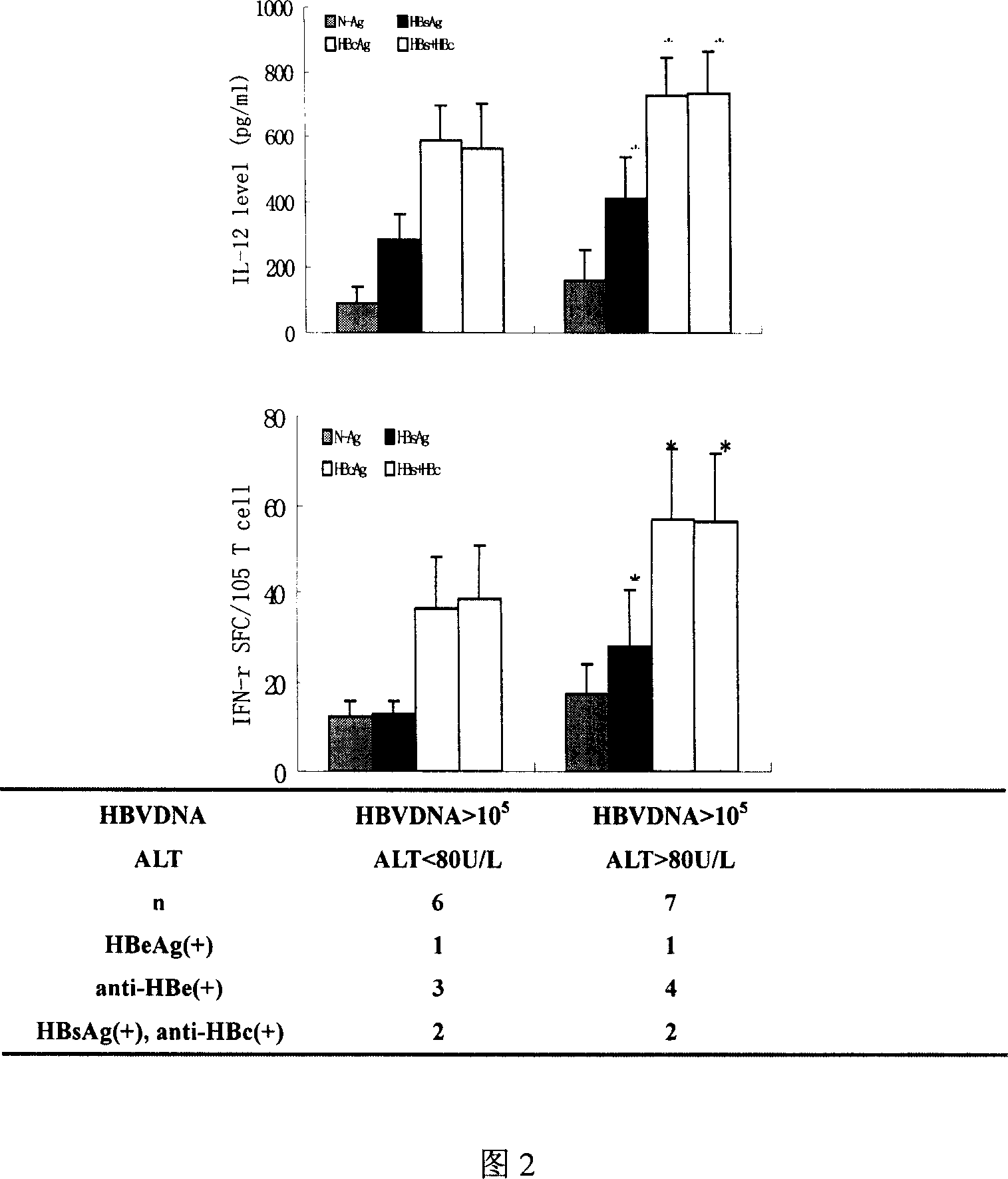
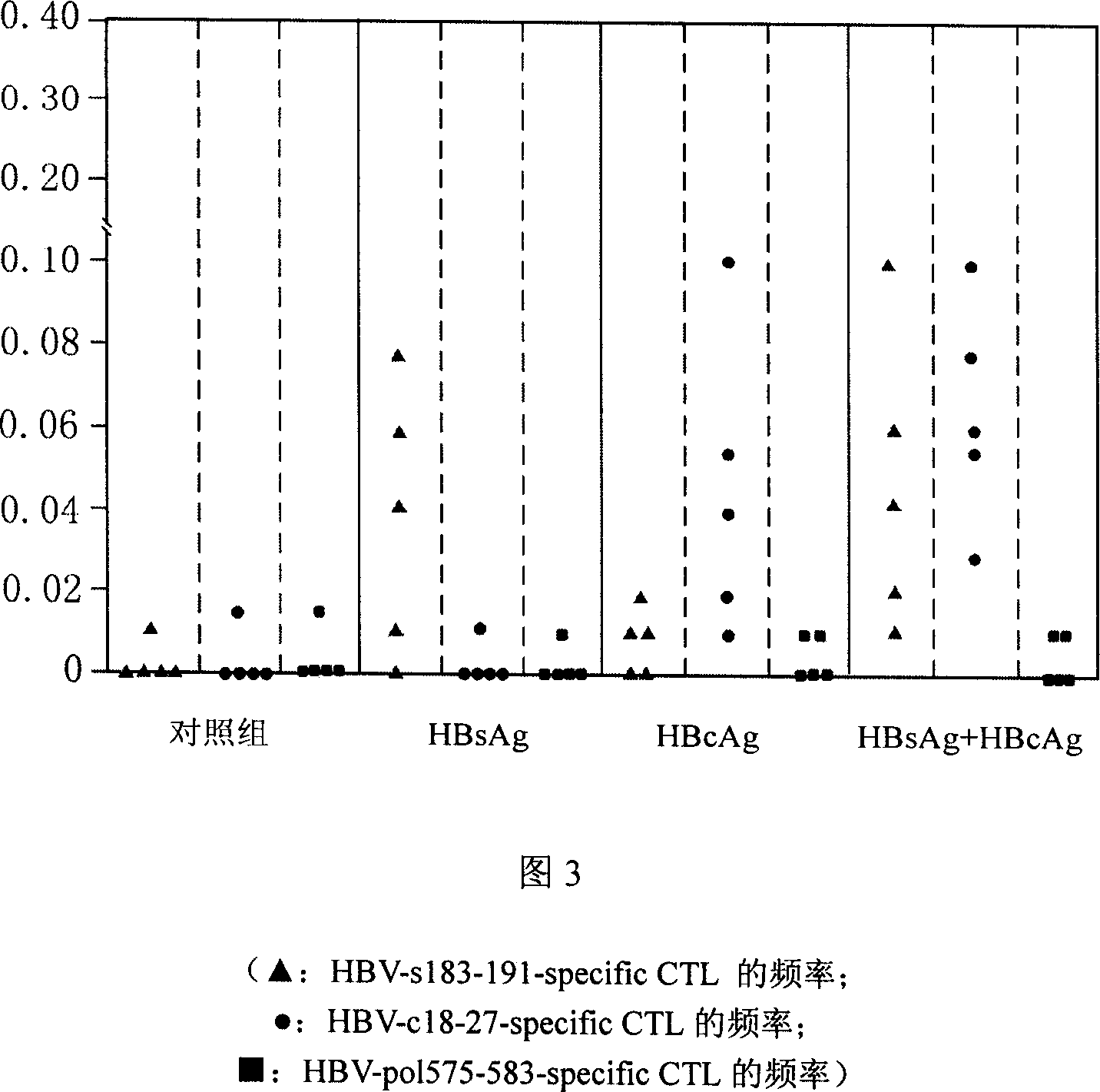
DC vaccine for treating chronic hepatitis B
A vaccine and specific technology, applied in the direction of antibody medical components, pharmaceutical formulations, antiviral agents, etc., can solve problems such as functional defects, low ability of T lymphocyte proliferation, and reduced number of proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The present invention is further illustrated by the following examples, but not as a limitation of the present invention. Embodiment 1, preparation
[0056] 1. The source of HBsAg and HBcAg: HBsAg comes from the semi-finished product of recombinant (yeast) hepatitis B vaccine without immune adjuvant. HBcAg is derived from the plasmid PMM2066 carrying the full gene sequence of HBcAg constructed in our laboratory, which is isolated and purified after expression in Escherichia coli.
[0057] 2. Isolation of PBMCs on day 0: Take 100ml of peripheral anticoagulant blood, separate PBMCs with lymphocyte separation medium, dilute the separated PBMCs to 20ml with pre-cooled PBS, divide them into two sterilized 10ml centrifuge tubes, and centrifuge Twice for 10 minutes to remove cell debris and platelets, and discard the supernatant. The PBMC depleted of cell debris and platelets were further diluted with PBS. Add the lymphocyte separation medium that has been returned to room ...
Embodiment 2
[0071] Embodiment 2: the preparation of injection
[0072] The vaccine obtained in step 7 or 9 of Example 1 was dissolved in water for injection according to the following formula, and prepared into injection according to conventional techniques.
[0073] Vaccine 1×10 7
[0074] Potassium chloride 0.04mg
[0075] Potassium dihydrogen phosphate 0.04mg
[0077] Disodium hydrogen phosphate .7H 2 O 0.432mg
[0078] The injection solution was prepared with distilled water to a final volume of 200 μl.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com