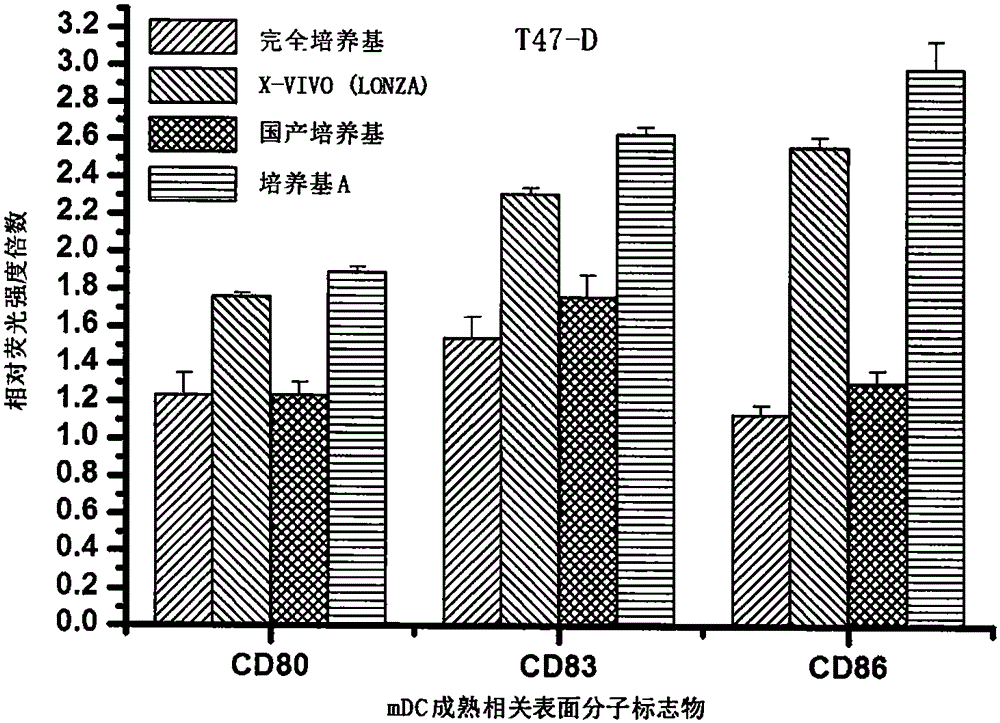
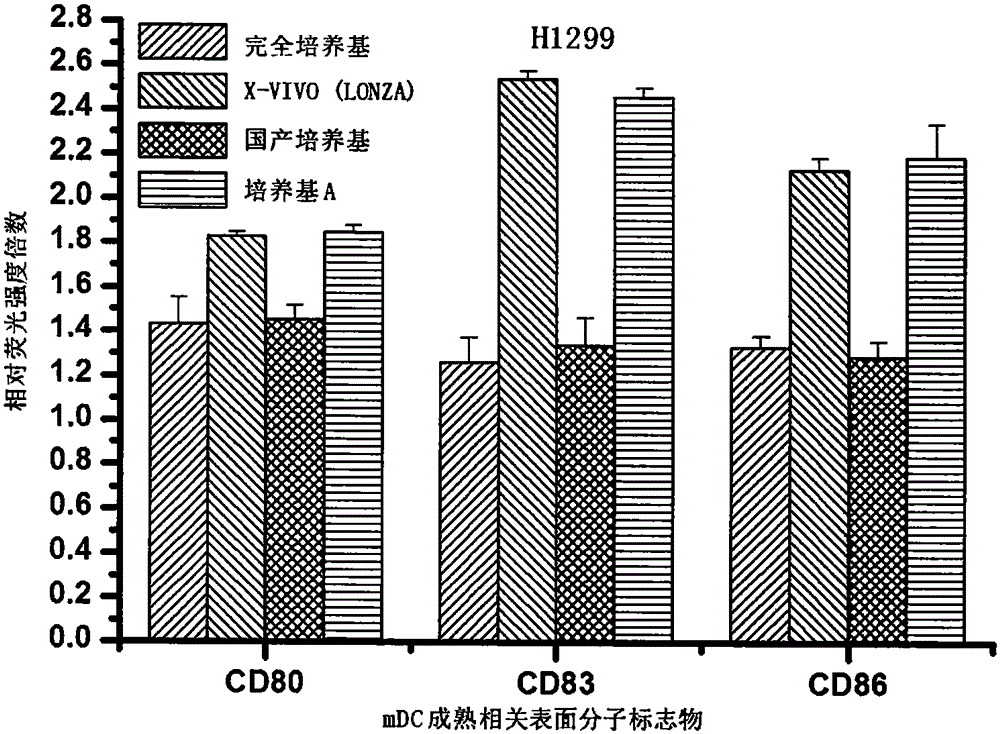
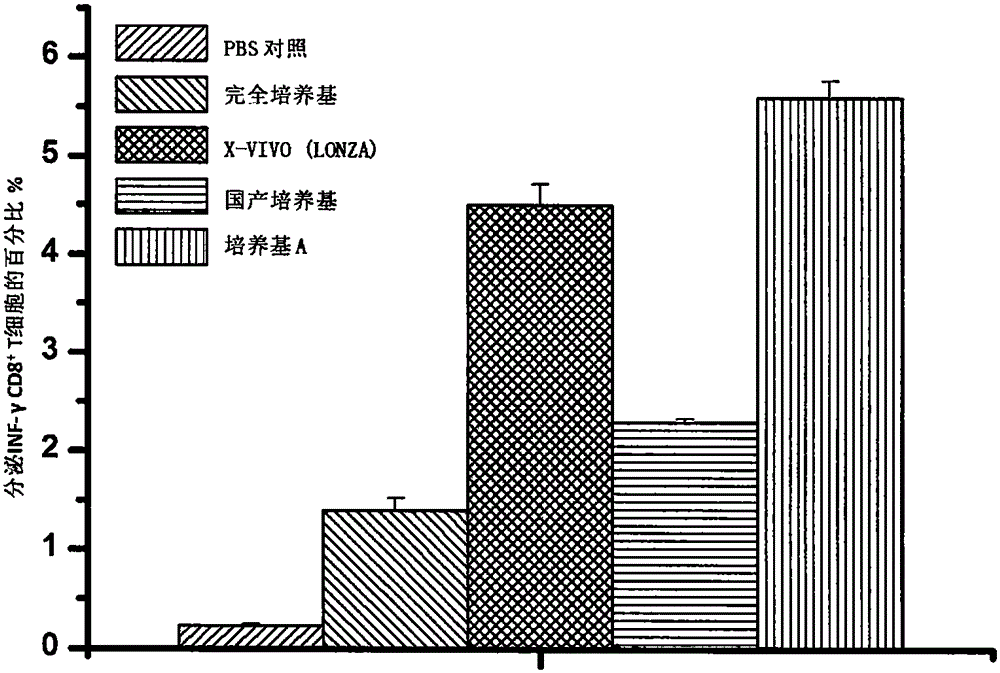
Antigen sensitized dendritic cell preparing method
A technology of dendritic cells and antigens, applied in the field of cell engineering and biomedicine, can solve the problems such as poor preservation and application of culture medium, failure to eliminate potential safety hazards of serum, and complex composition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of medium A for the preparation of antigen-sensitized dendritic cells
[0052] The medium used for the preparation of antigen-sensitized dendritic cells of the present invention consists of basic medium and other components. Preferably, the basal medium is RPMI1640 medium, and the other components are composed of formula A, wherein formula A is shown in Table 1.
[0053] Table 1 is used for formula A prepared by antigen-sensitized dendritic cells
[0054]
[0055]
[0056] Among them, pharmaceutical grade human albumin, recombinant human insulin, recombinant human fibronectin and rhIL-4 were purchased from R&Dsystem, and the other reagents were purchased from Sigma-Aldrich Company. Described formula A preparation method is as follows:
[0057] 1. Preparation of cholesterol solution: Weigh 50 mg of cholesterol powder, add it to 20 ml of RPMI1640 medium, shake it with an ultrasonic oscillator at 4°C for 1 hour to emulsify it completely, and obt...
Embodiment 2
[0061] Example 2 Utilizing HHP to Prepare Tumor Antigen with Strong Immunogenicity
[0062] 1. Human breast cancer cells T47-D and human non-small cell lung cancer cells H1299 were respectively selected to be treated with HHP (the tumor cell lines were all purchased from the Institute of Basic Medical Sciences, Peking Union Medical College, China), and the treatment conditions were HHP=250MPa, and the treatment temperature was 25 ℃, the treatment time is 20 minutes. After treatment, the cells were cultured with RPMI1640 containing 10% FBS for 48 hours at 37° C., 5% CO 2 , and saturated humidity.
[0063] 2. The cell density in the obtained cell sample is 10 6 / ml, the storage volume is 1ml, resuspended in serum-free freezing solution (CryostorCS10, Biolife Solutions, USA), and frozen in liquid nitrogen.
Embodiment 3
[0064] Example 3 Using medium A of the present invention to induce PBMC-derived iDC cells
[0065] 1. Add 5ml of cell separation medium (human mononuclear cell separation medium 1.077, product number 25710, Beijing Dongfang Huahui Biomedical Technology Co., Ltd.) into a 15ml centrifuge tube (SARSTEDT, Germany), and then add 5ml of PBS buffer Biomedical Technology Co., Ltd.) 1:1 diluted peripheral blood samples were carefully spread on the cell separation medium along the tube wall, and centrifuged for 15 minutes at a temperature of 20° C. and a centrifugation speed of 2000 rpm.
[0066] 2. After centrifugation is stopped, PBMCs form a relatively obvious cloud layer at the interface between the separation liquid and plasma. Use a pipette to suck out the serum layer and discard it, and then use a pipette to carefully suck out the cloudy cell strips and place them in another Into a centrifuge tube, add 5 times the volume of Hank'S solution (Beijing Dongfang Huahui Biomedical Tech...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com