Chronic hepatitis B treatment DC vaccine
A chronic hepatitis B, therapeutic technology, applied in the field of biomedicine, can solve problems such as inability to produce therapeutic effects, and achieve the effects of improving efficiency, high infection rate, and enhancing immune effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The specific implementation is as follows:
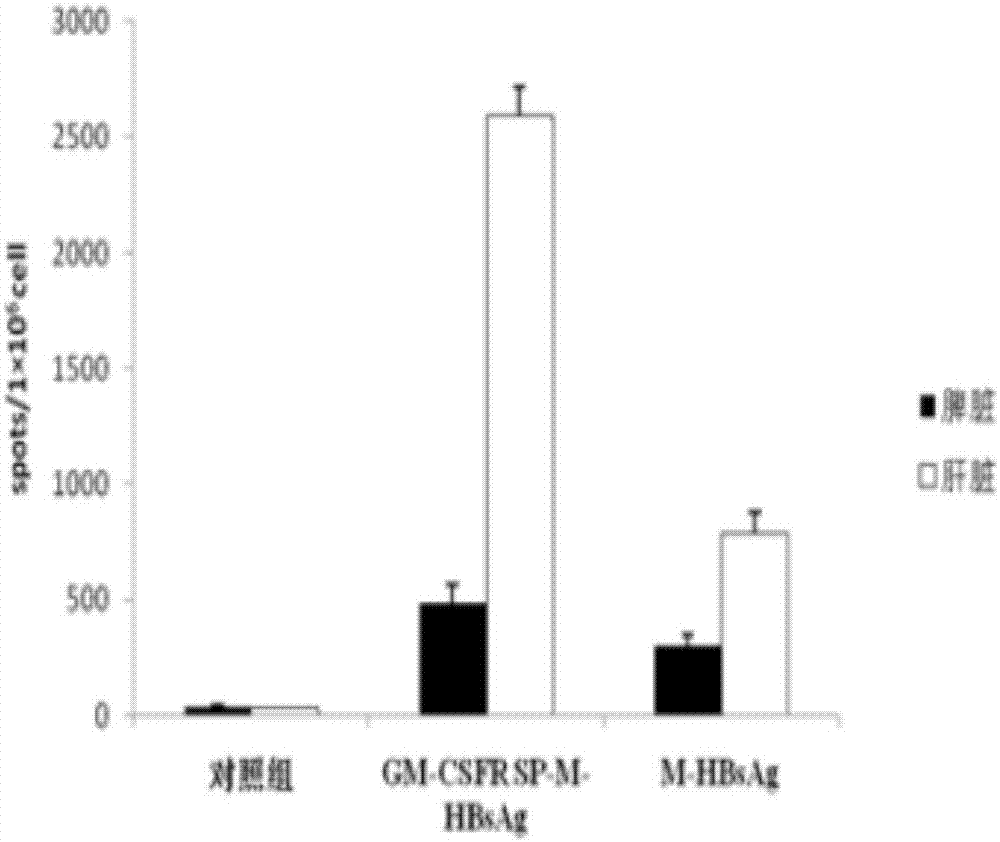
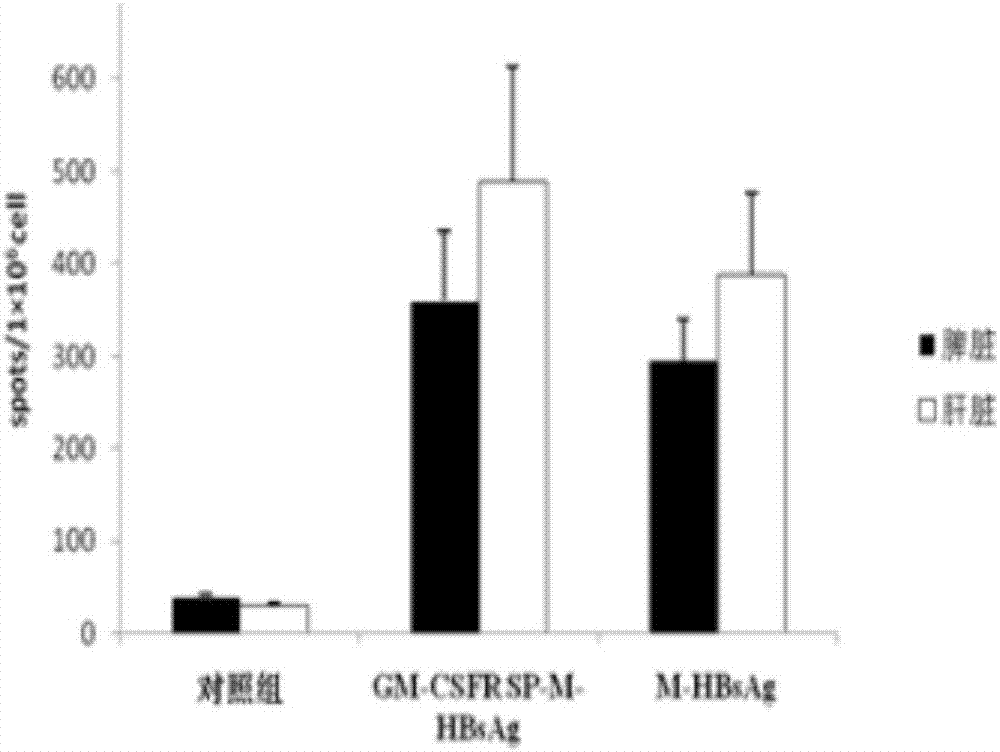
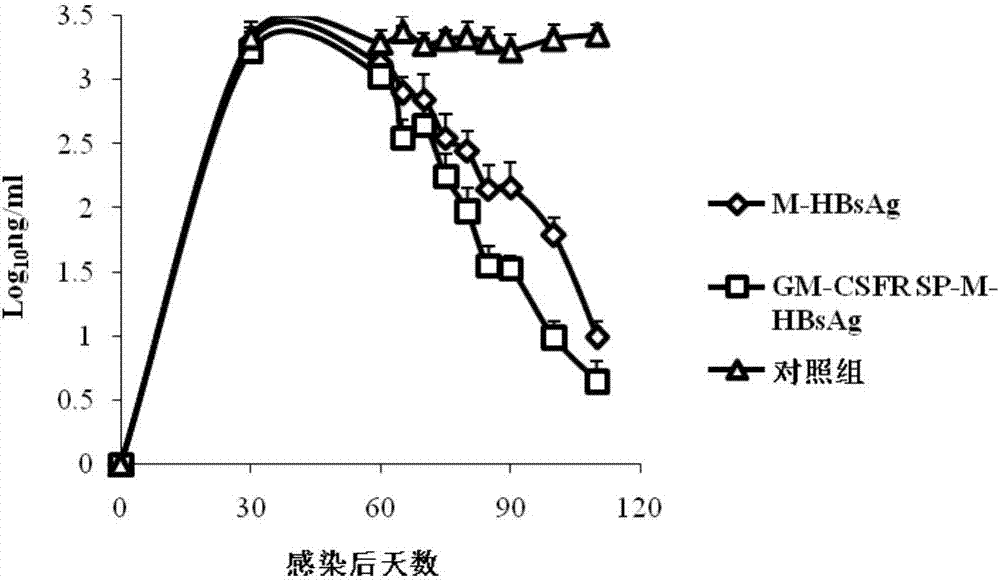
[0024] 1. Insert the gene fragment GM-CSFR SP-M-HBsAg into the lentiviral expression vector pLent-C-GFP.
[0025] (1) GM-CSFR SP nucleic acid artificial sequence (SEQ ID NO.1)
[0026] (2) M-HBsAg nucleic acid artificial sequence (SEQ ID NO.2)
[0027] The two ends of the nucleic acid artificial sequence of GM-CSFR SP-M-HBsAg were respectively added with NotI and AsiSI site enzyme cutting sites (SEQ ID NO.4), and entrusted Sangon Bioengineering Co., Ltd. to synthesize two complete expression cassettes. After cleavage, insert the NotI-AsiSI site of the lentiviral pLent-C-GFP vector (Invitrogen), transform into E.coli (DH5α), and after the correct identification by nucleic acid sequencing, use the plasmid purification kit from Qiagen to extract and purify the plasmid. Obtain high-quality plasmids for recombinant expression vectors.
[0028] 2. Recombinant lentivirus packaging, titer detection
[0029] Inoculate the lentivir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com