Method for production of dendritic cell
一种树突状细胞、细胞的技术,应用在生物化学设备和方法、动物细胞、脊椎动物细胞等方向,能够解决无法达到理想治疗效果、不能获取DC、DC前体细胞数量有限等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0206] The present invention will be further described below through examples, but the present invention is not limited to these examples. In addition, all the documents cited in this specification are incorporated as a part of this specification.
[0207] The composition components of the FS36 administration group, the GMSCF administration group and the GMIL-4 administration group in Examples 1, 2, 4, 5 and the figures of the examples are as follows.
[0208] FS36 administration group: containing Flt-3 ligand (20ng / ml), stem cell factor (Stem cell factor; SCF) (10ng / ml), IL-3 (10ng / ml), IL-6 (10ng / ml) ( Abbreviated as FS36), RPMI1640 with 10% FBS.
[0209] GMIL-4 administration group: RPMI1640 containing GM-CSF (20 ng / ml), IL-4 (20 ng / ml), and 10% FBS.
[0210] GMSCF administration group: RPMI1640 containing GM-CSF (20 ng / ml), SCF (10 ng / ml), and 10% FBS.
[0211] The GMIL-4 administration group (1), the GMIL-4 administration group (2), the GMSCF administration group, the ...
Embodiment 6
[0217] (1) iDC treatment, (2) SeV / dF treatment, and (3) LPS treatment in Example 6 and the drawing of the example are as follows.
[0218] (1) iDC treatment: Incubate for 2 days in the medium at the following concentrations.
[0219] IMDM with 10% FBS
[0220] (2) SeV / dF treatment: Incubate for 2 days in the medium at the following concentrations.
[0221] IMDM containing F gene deletion Sendai virus (moi=50), 10% FBS
[0222] (3) LPS treatment: Incubate for 2 days in the medium at the following concentration.
[0223] IMDM containing LPS (1 μg / ml), 10% FBS
[0224] This experiment uses the following LPS.
[0225] SIGMA Catalog No.L7895-1MG (biological source = Salmonella typhosa)
[0226] (4) Poly(I:C) treatment: Incubate for 2 days in the medium at the following concentration.
[0227] IMDM containing Poly(I:C) (100 μg / ml), 10% FBS
[0228] (5) CpG treatment: Incubate for 2 days in the medium at the following concentration.
[0229] IMDM containing CpG (10 μg / ml), 10...
Embodiment 1
[0234] [Example 1] Confirmation of Dendritic Cell (DC) Progenitor Expansion and Differentiation of Cytokines
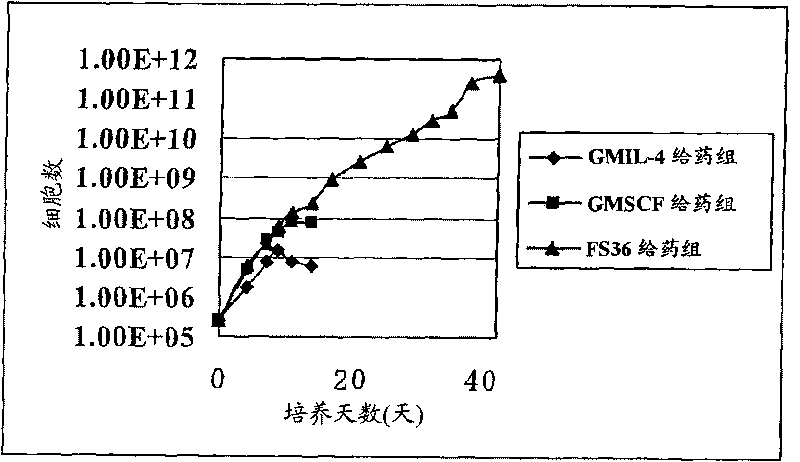
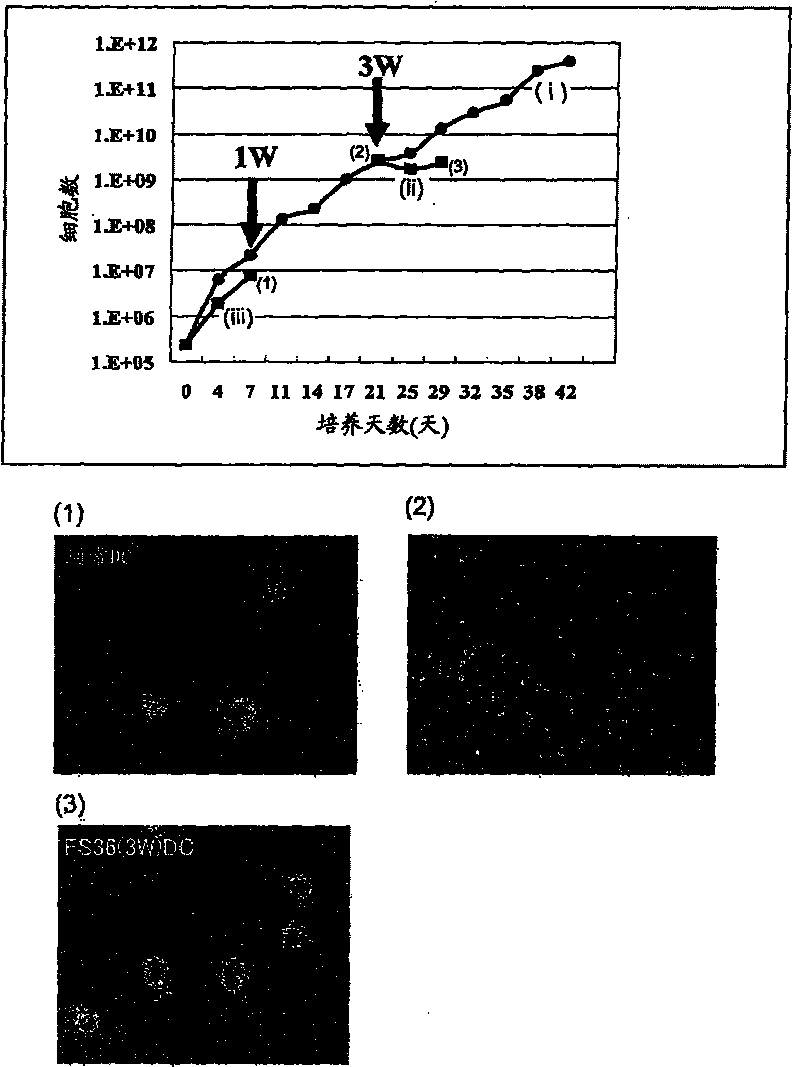
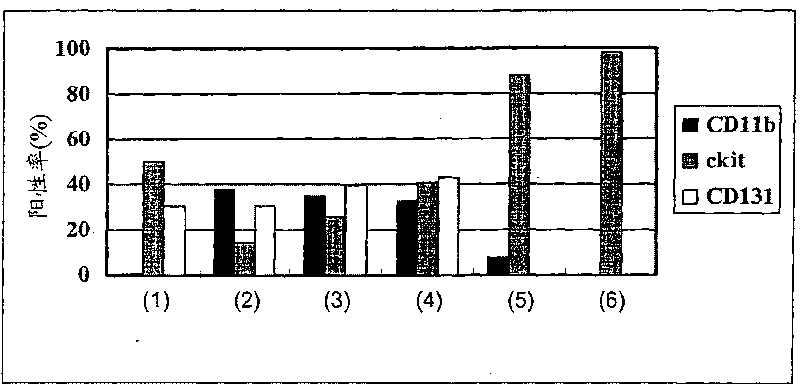
[0235] First, hematopoietic precursor cells (SpinSep mouse hematopoietic progenitor enrichment kit, StemCelltechnologies, Canada) were extracted from mouse (C3H) femur and tibia bone marrow by negative selection. The precursor cells were divided into three groups including FS36 administration group, GMIL-4 administration group and GMSCF administration group for culture. cultured by 2.4 x 10 5 From the beginning, the cells were subcultured every 3 to 4 days at a concentration of less than 2 million cells / ml for 6 weeks. Modulation of dendritic cell (DC) precursor cells during culture ( figure 1 ), count the number of cells, confirm the rate of expansion, and perform FACS analysis after staining with anti-CD11b-FITC, anti-CD11c-PE, anti-c-kit-PE, and anti-CD131-PE, and confirm the differentiation ability of the above-mentioned precursor cells ( image 3 ).
[0236] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com