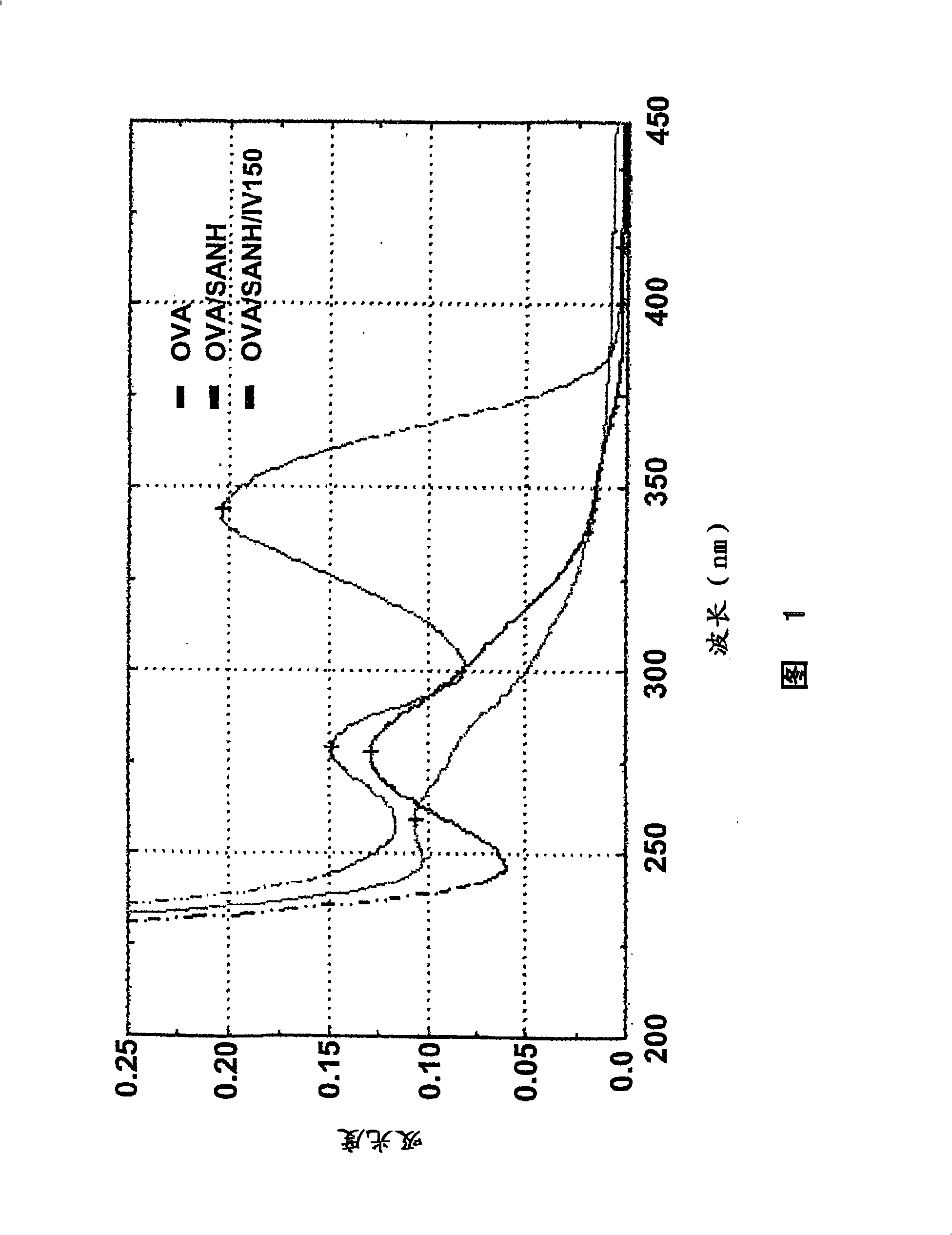
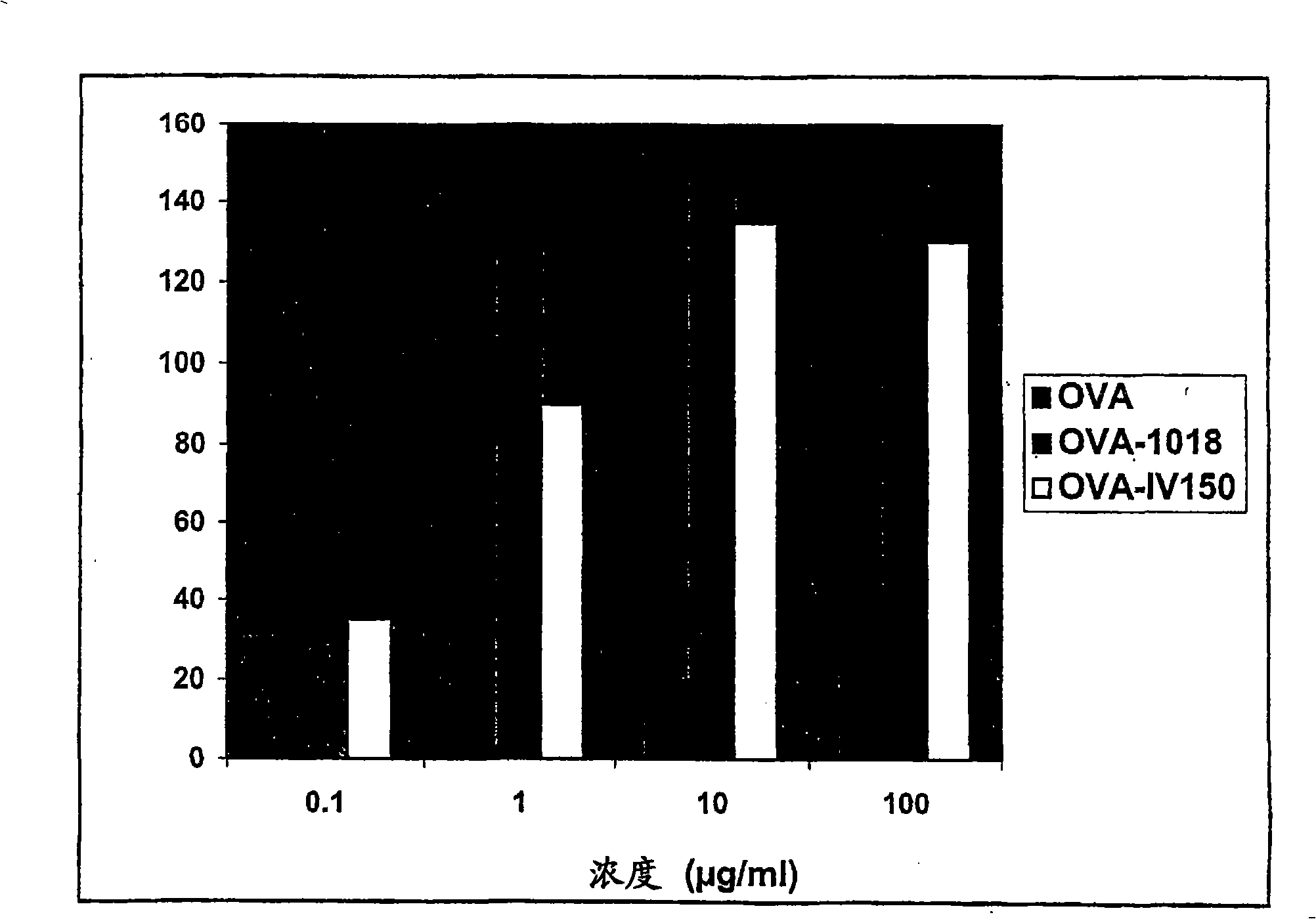
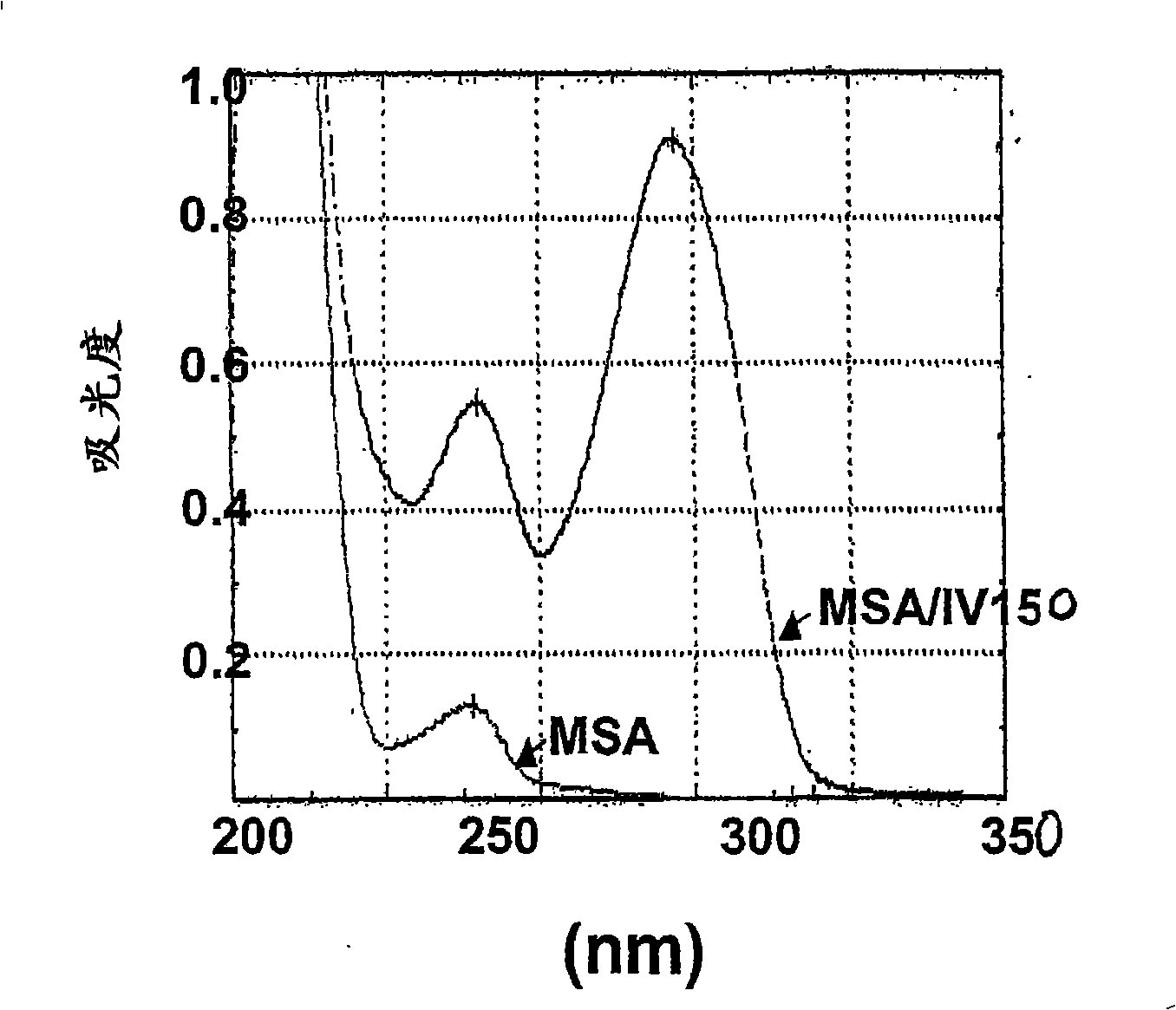
Tlr agonists
A compound and alkyl technology, which can be used in antiviral agents, allergic diseases, antibacterial drugs, etc., and can solve the problem of reducing auxiliary effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Example 1 The preparation of 4-(2,6-dichloropurin-9-ylmethyl)benzonitrile
[0155] 2,6-Dichloro-9H-purine (16 mmol) was dissolved in DMF (50 mL) and potassium carbonate (50 mmol) was added. α-Bromo-p-tolylcyanide (22 mmol) was then added, and the mixture was stirred at room temperature for 16 h. After removal of insoluble inorganic salts by filtration, the filtrate was poured into water (1500 mL) and extracted with ethyl acetate (2 x 400 mL), dried over magnesium sulfate and evaporated to give a residue which was obtained by using 1:2:10 ethyl acetate Flash chromatography on silica gel of esters / acetone / hexanes. Yield 3.33 g (69%). UV, NMR and MS were consistent with the assigned structure.
Embodiment 2
[0156] Example 2 Preparation of 4-(6-amino-2-chloropurin-9-ylmethyl)benzonitrile
[0157] The product of Example 1 (1.9 g) was placed in a steel reaction vessel and methanolic ammonia (80 mL, 7N) was added. The sealed vessel was heated at 60 °C for 12 h, cooled in ice and the solid product was filtered off. Yield 1.09 g. UV, NMR and MS were consistent with the assigned structure.
Embodiment 3
[0158] Example 3 Preparation of 4-[6-amino-2-(2-methoxyethoxy)purin-9-ylmethyl]benzonitrile
[0159] The sodium salt of 2-methoxyethanol was produced by dissolving metallic sodium (81 mg) in 2-methoxyethanol (30 mL) with heating. To this solution was added the product of Example 2 (1.0 g) dissolved in methoxyethanol (300 mL, heated). The reaction mixture was heated at 115 °C bath temperature for 8 h, concentrated in vacuo to near dryness, and the residue was partitioned with ethyl acetate and water. The organic layer was purified by flash chromatography on silica gel using 5% methanol in dichloromethane to afford 763 mg of product. NMR was consistent with the assigned structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com