siRNA echovirus delivery system with shell-core structure and application of system
A technology of delivery system and core structure, which is applied in the field of siRNA imitation virus delivery system with shell-core structure, can solve the problems of poor escape ability of endosome, slow intracellular release, limited compression ability, etc., and achieve excellent active targeting performance, High gene load, reduced binding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 The preparation of the siRNA imitation virus delivery system of the shell-core structure of the present invention
[0038] The c(RGDyK) used in the examples of the present invention was purchased from Gill Biochemical (Shanghai) Co., Ltd.
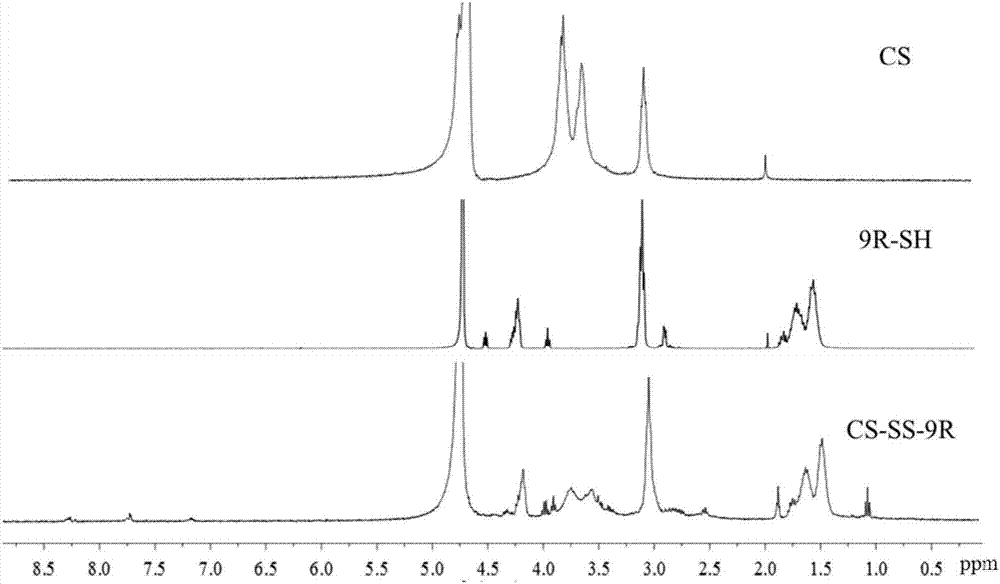
[0039] 1. Preparation and characterization of core and shell materials
[0040] (1) Core material: preparation of chitosan-disulfide bond-nona-arginine (CS-SS-9R)
[0041] 10 mg of chitosan (CS, MW: 10000) was dissolved in 4 mL of deionized water, and the pH value was adjusted to 6.0 with 2% triethylamine. 9.6 mg of N-succinyl-3-2-dithiopyridine-propionate (SPDP) was dissolved in 4mL DMSO, the CS solution was slowly added dropwise to the SPDP solution, and the reaction was performed under magnetic stirring at room temperature for 24h. The reaction solution was dialyzed with deionized water for 48 hours to remove unreacted raw materials and by-products. Subsequently, 1 mL of 9.5 mg / mL nona-arginine (9R-SH) was added to...
Embodiment 2
[0053] Example 2 Investigating the ability of the siRNA imitation virus delivery system of the present invention to carry siRNA
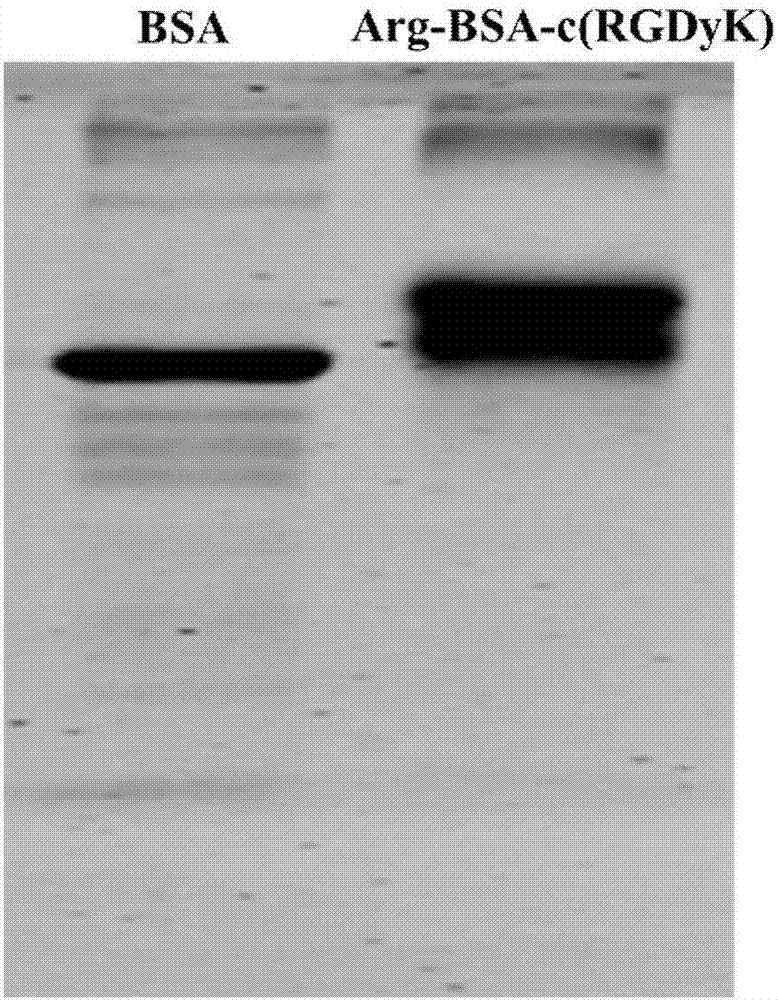
[0054] The mass ratios of CS-SS-9R and siRNA were selected as 5:1, 10:1, and 20:1, and the mass ratios of CS, 9R and siRNA were both 5:1. Dissolve siRNA in DEPC-treated water, add corresponding doses of CS, 9R, and CS-SS-9R solutions dropwise, vortex for 30 seconds, and place at room temperature for 30 minutes. Gel retardation experiments were used to investigate the binding ability of CS, 9R and CS-SS-9R to siRNA, the results are shown in Figure 4 . The bands condensed in the wells in the figure indicate that the binding ability between the carrier and siRNA is strong, and the migrating bands indicate that the binding ability is weak.
[0055] The results showed that the combination of chitosan and nona-arginine increased the binding capacity of siRNA. When the weight ratio of inner core material to siRNA was 5:1, the blocking ability of CS-SS-...
Embodiment 3
[0057] Example 3 Investigating the GSH-responsive release ability of the siRNA imitation virus delivery system of the shell-core structure of different core materials
[0058] Taking the core material CS-9R as a comparative example, the GSH-responsive release ability of the siRNA imitation virus delivery system with shell-core structure of different core materials was investigated.
[0059] 1. Preparation of CS-9R / siRNA / Arg-BSA-c(RGDyK):
[0060] Preparation of CS-9R: Dissolve 10mg chitosan (MW: 10000) in 4mL water, adjust the pH value to 6.0 with 2% triethylamine, dissolve 13.5mg Sulfo-SMCC in 2mL water, add the Sulfo-SMCC aqueous solution dropwise to the CS solution , the reaction was performed under magnetic stirring for 24 h at room temperature, and the by-products were removed by dialysis for 24 h. Subsequently, 9.5 mg of 9R-SH was added to the dialysate, reacted under magnetic stirring at room temperature for 24 hours, dialyzed in deionized water for 72 hours to remove ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com