Immunostimulatory nucleic acid molecules for activating dendritic cells
a technology of dendritic cells and nucleic acids, applied in the field of oligonucleotides, to achieve the effects of less toxic, less favorable th2 response, and higher th1 respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation and Characterization of Dendritic Cells
Methods
[0122] Isolation of dendritic cells: Dendritic cells represent a small population of peripheral blood mononuclear cells (0.1 to 0.4%). They express substantial levels of CD4, but lack the T cell molecules CD3, CD8, and T cell receptor, and other lineage markers (CD19, CD14, CD16, CD56) (O'Doherty U, et al., “Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium”, J Exp Med, 1993; 178: 1067-1076). Using these characteristics, dendritic cells can be separated by high gradient immunomagnetic cell sorting using the VARIOMACS technique (Miltenyi Biotec Inc., Auburn, Calif.). Peripheral blood mononuclear cells were obtained from buffy coats of healthy blood donors (Elmer L. DeGowin Blood Center, University of Iowa) by Ficoll-Paque density gradient centrifugation (Histopaque-1077, Sigma Chemical Co., St. Louis, Mo.) as de...
example 2
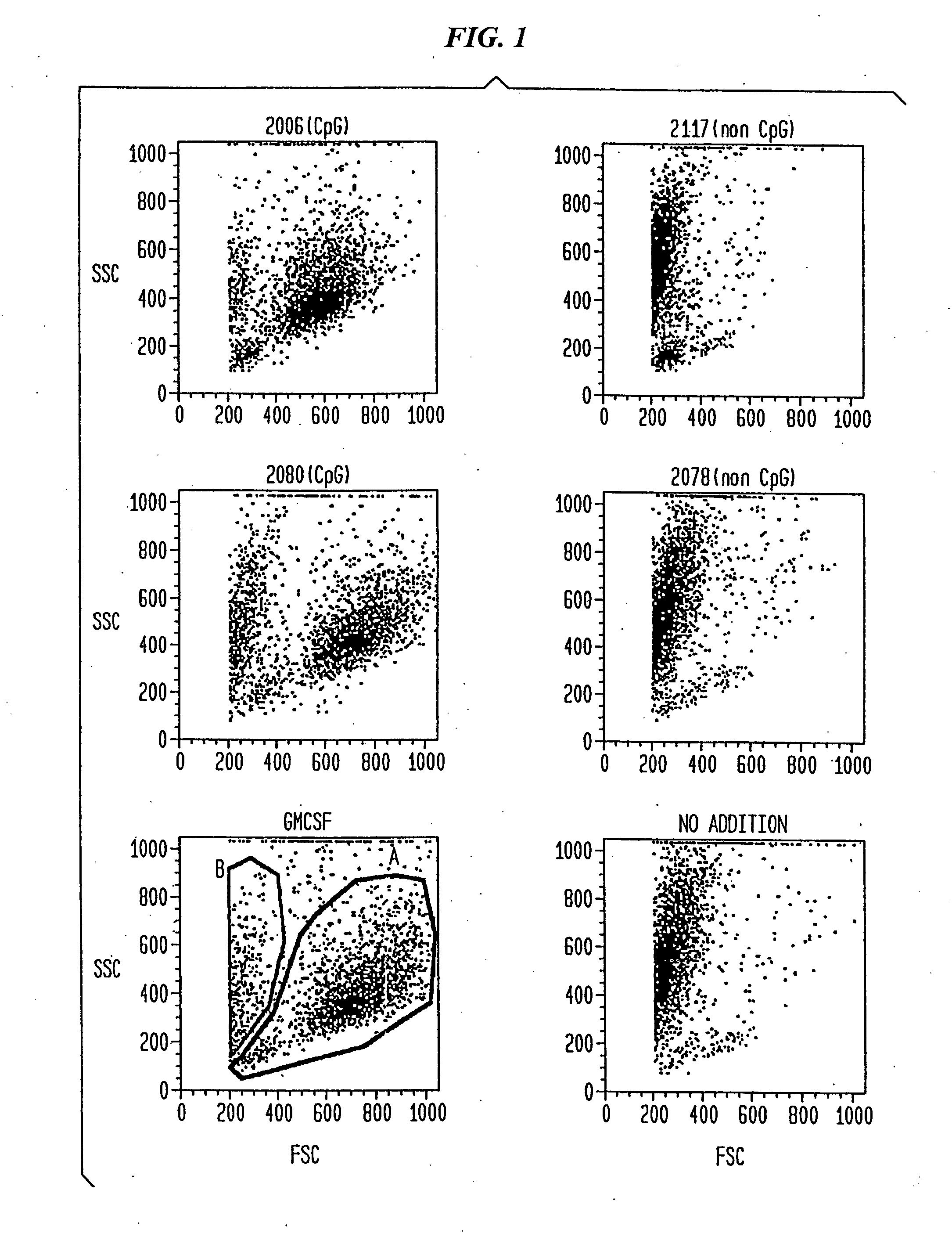
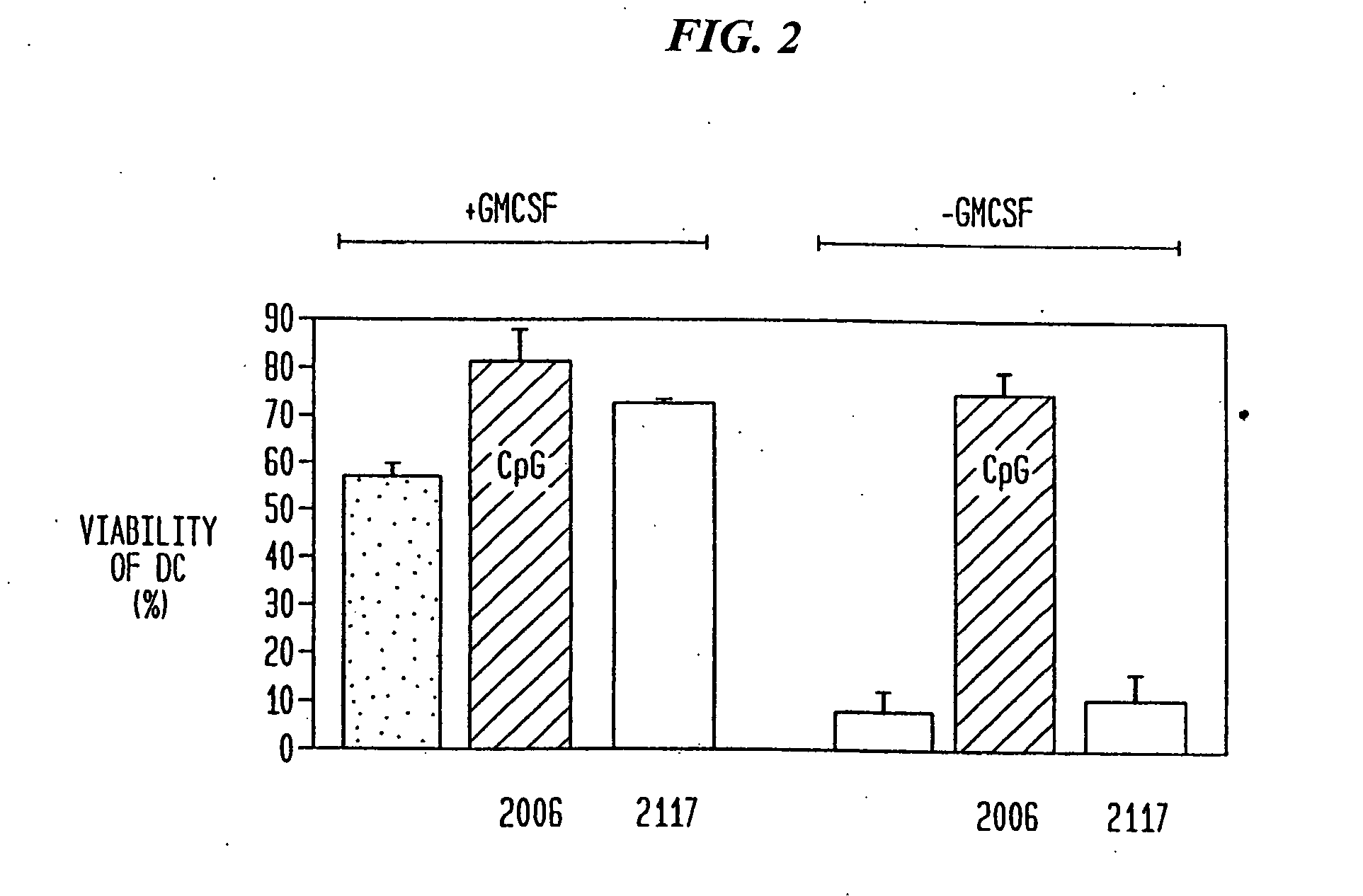
CpG Substitutes for GMCSF for DC Survival
Methods
[0126] Oligodeoxynucleotides Unmodified (phosphodiester) and modified nuclease-resistant (phosphorothioate) oligodeoxyribonucleotide were purchased from Operon Technologies (Alameda, Calif.). The optimal motif recognized by human immune cells is different from the optimal mouse motif. Based on other studies in which we tested a large number of oligonucleotides for their ability to activate human B-cells and NK-cells, we selected particularly potent oligonucleotides as examples of a family of active CpG-containing oligonucleotides for the use in the present study. The CpG oligonucleotides used were: 2006 (24-mer), 5′-TCG TCG TTT TGT CGT TTT GTC GTT-3′ (SEQ ID NO: 84), completely phosphorothioate-modified, and 2080 (20-mer), 5′-TCG TCG TTC CCC CCC CCC CC-3′ (SEQ ID NO: 94), unmodified phosphodiester. The non-CpG control oligonucleotides used were: 2117 (24-mer), 5′-TQG TQG TTT TGT QGT TTT GTQ GTT-3′ (SEQ ID NO: 95), Q=5 methyl cytosin...
example 3
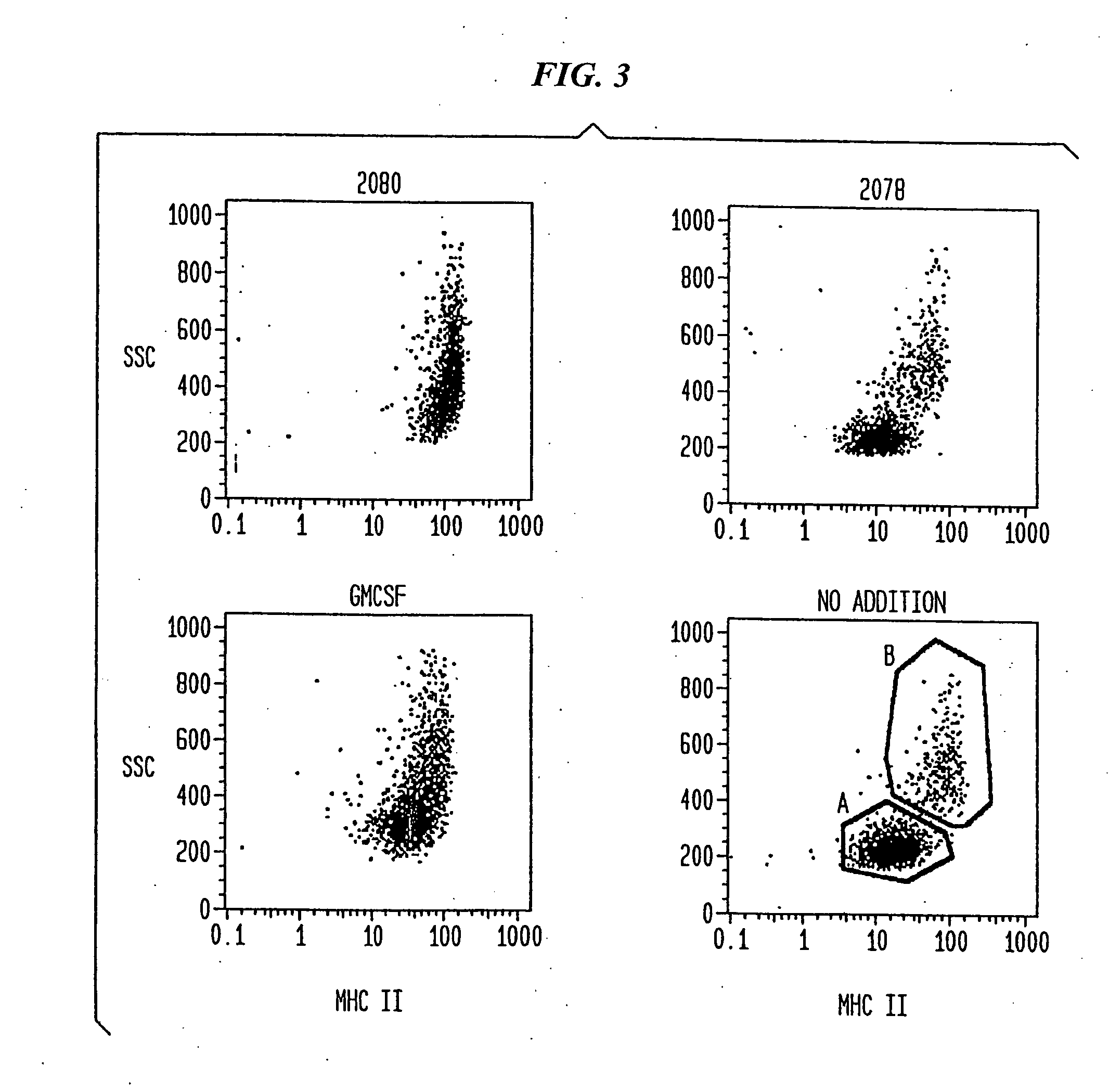
Increased Size and Granularity of DC Induced by CpG is Associated with Enhanced Expression of MHC II
Methods
[0130] Surface antigen staining At the indicated time points, cells were harvested and surface antigen staining was performed as previously described. Monoclonal antibodies to HLA-DR (Immu-357), CD80 (MAB104) and to CD83 (HB15A) were purchased from Immunotech, Marseille, France. All other antibodies were purchased from Pharmingen, San Diego, Calif.: mABs to CD1a (HI149), CD3 (UCHT1), CD14 (M5E2), CD19 (B43), CD40 (5C3), CD54 (HA58), CD86 (2331 (FUN-1)). FITC-labeled IgG1, κ (MOPC-21) and PE-labeled IgG2b, κ (27-35) were used to control for specific staining.
Results
[0131] Flow cytometric analysis suggested that differentiation of DC is enhanced by CpG and is associated with an increase of cell size (FSC) and granularity (SSC) (FIG. 1). The surface expression of MHC II is known to be positively correlated with differentiation of DC. DC isolated from peripheral blood were cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com