Method for testing efficacy of swine fever live vaccine
A swine fever live vaccine and inspection method technology, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, measuring devices, etc. Uniformity, significant differences in experimental results, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Test method for efficacy of swine fever live vaccine
[0032] 1. Preparation of Cells for Passaging
[0033] The porcine testis (ST) cells (derived from ATCC, American Type Culture Collection) covered with a single layer were digested and dispersed with 0.25% trypsin (containing 0.02% EDTA) and then added with 3% fetal bovine serum α-MEM cells were maintained in culture medium, and after cell counting, the cell suspension was added to a 96-well cell plate, 100 μl per well.
[0034] 2. Dilution of live swine fever vaccine
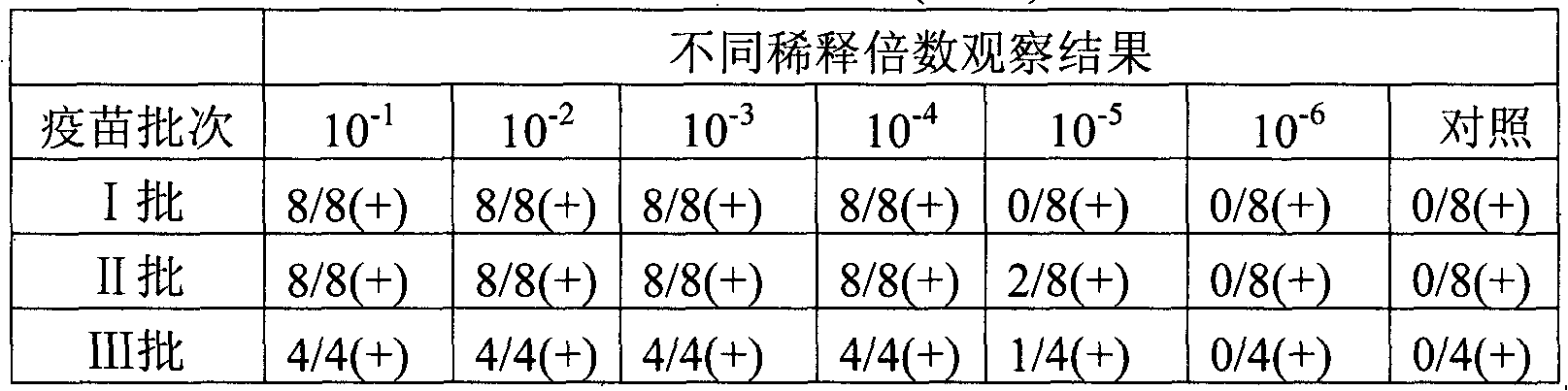
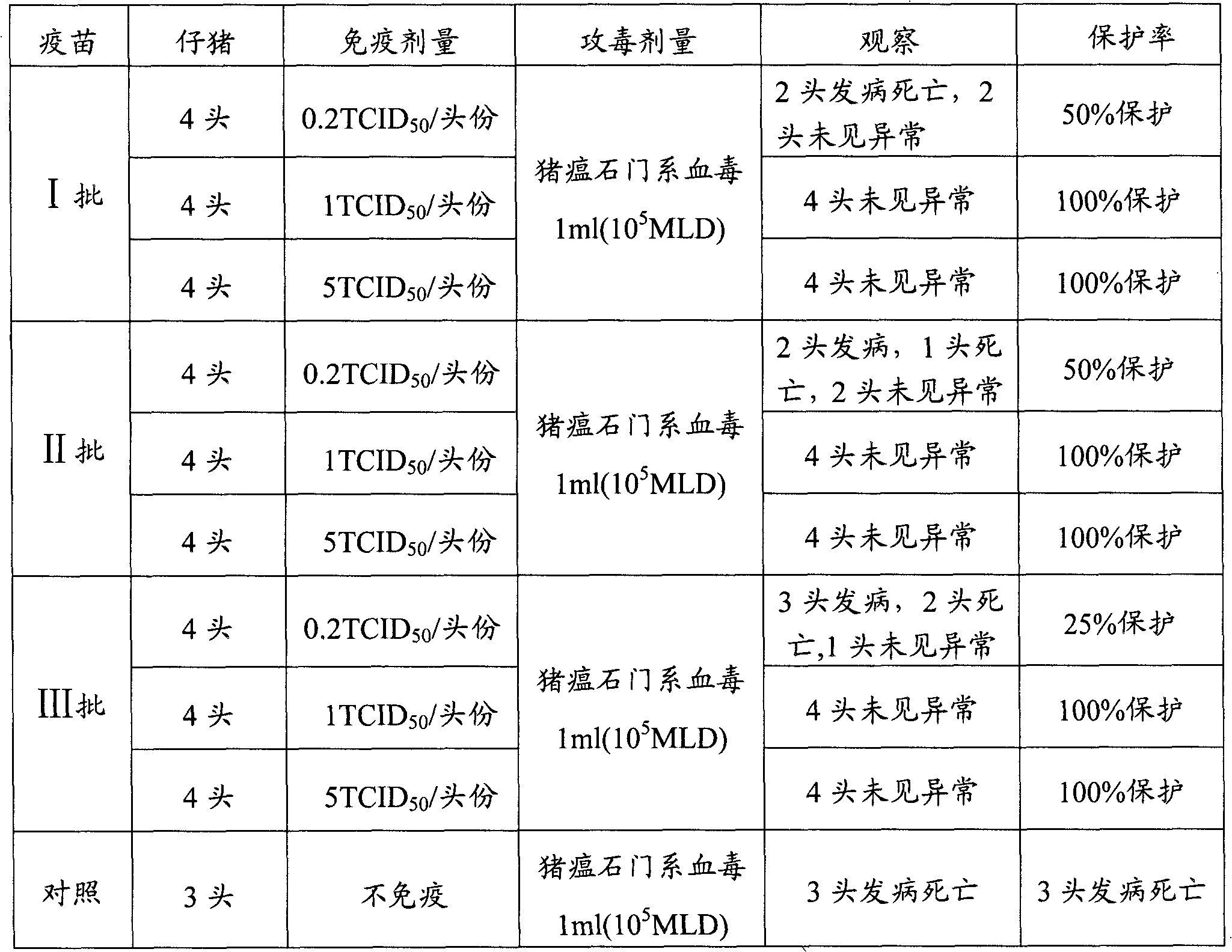
[0035] In this example, three batches of live vaccines were prepared, and their antigen contents were determined respectively.
[0036] The strain of the swine fever live vaccine is an attenuated strain of swine fever lathe (preserved by China Veterinary Drug Administration, preservation number AV1412). Inoculate ST cells with attenuated CSF rabbitized strain, and mix the harvested virus antigen solution with appropriate lyoprotectant to make CSF l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com