Nucleic acid vaccine composition comprising a lipid formulation, and method of increasing the potency of nucleic acid vaccines
a technology of nucleic acid and composition, which is applied in the direction of dna/rna vaccination, viruses/bacteriophages, antibody medical ingredients, etc., can solve the problems of insufficient potency of vaccines, inability to produce exceptionally high levels of immunogen, etc., to enhance the potency of plasmid-based dna vaccines and immunotherapies, increase the level of immunogen or immune response molecules, and improve the effect of vaccine potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on with Unformulated DNA Vaccine
[0036]In previous studies, rabbits were vaccinated with unformulated Andes DNA vaccine and the following results were obtained:
[0037]Study 1 (Unformulated DNA).
[0038]Eight rabbits were vaccinated with unformulated Andes DNA vaccine using PharmaJet IM, 2 mg / injection. After 1 vaccination (Week 4) PsVNA80=81, 40, 57, <20, <20, <20, 43, 34. GMT=27 (when <20 are given value of 10).
[0039]Study 2 (Unformulated DNA).
[0040]A preclinical toxicity study in rabbits was performed. Eight rabbits received 4 vaccinations using unformulated Andes DNA vaccine, 2 mg / injection×4 injections per vaccination (a total of 32 mg of DNA). The PsVNA80 after 3 vaccinations was GMT=7,781 (Lower 95% 5,061, Upper 95% 11,966) and after 4 vaccinations was 18,899 (Lower 95% CI 7,181, Upper 95% CI 49,738) (J. Hooper, Contributing Scientist Report AN-8327478-G, unpublished). This represents the predicted maximum neutralizing activity that can be produced in rabbits vaccinated with unfor...
example 2
on with DNA Vaccine Formulated with the Inventive Lipid Formulation
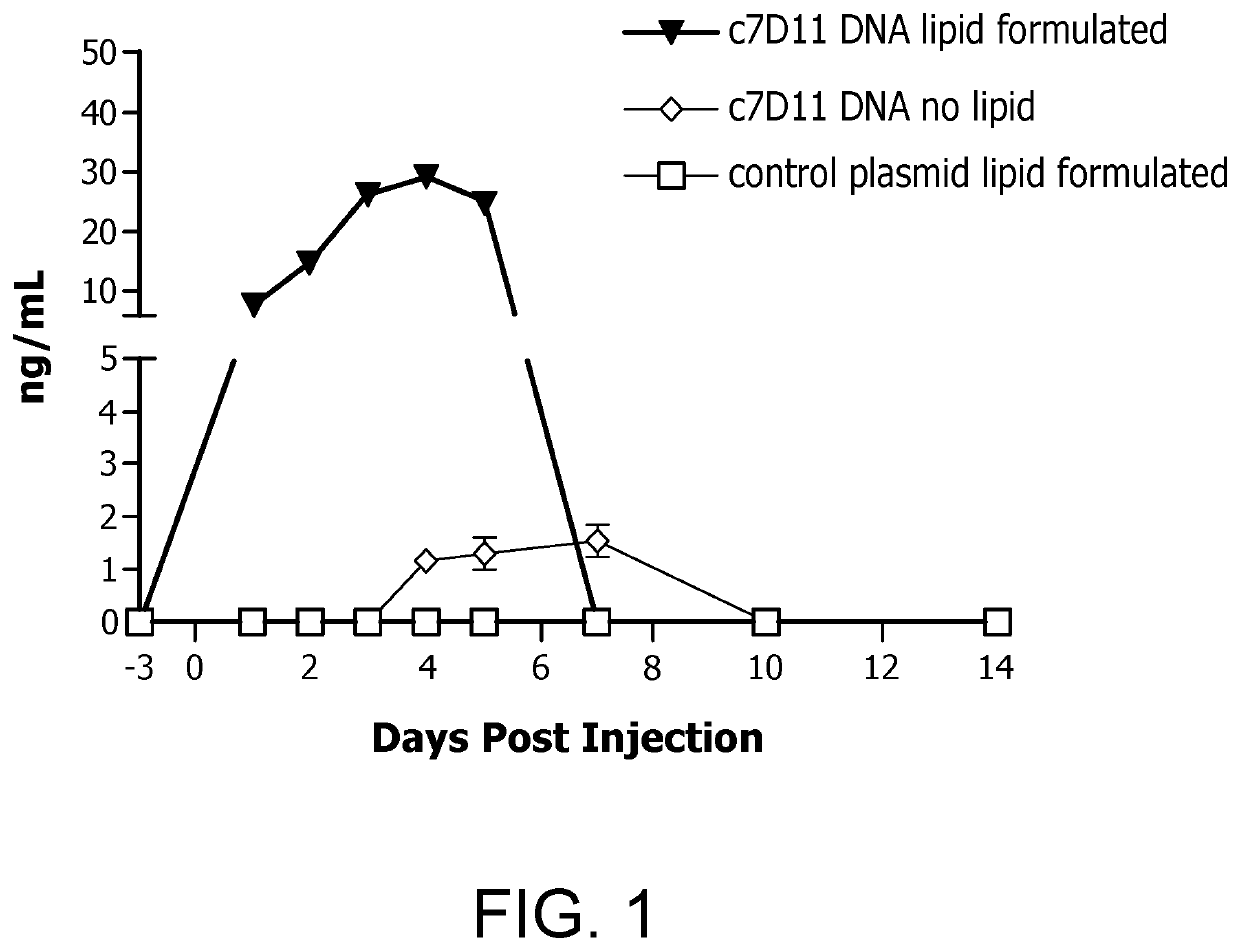
[0041]The present inventors hypothesized that the lipid formulation disclosed herein may increase the potency of nucleic acid vaccines. To evaluate this possibility, testing was performed on a rabbit, wherein the rabbit was given a single intramuscular injection (PharmaJet Stratis) of a vaccine containing 1 mg of DNA vaccine plasmid pWRG / c7d11 (H+L) combined with the lipid formulation of the present invention. Sera specimens were collected before injection (Day-4, relative to injection) and after (Days 1-5, 7, 10, 14 and 21, relative to injection). Recombinant monoclonal human antibody was detected and quantified from the sera by an immunogen specific ELISA. The data is presented with historical controls (n=4) in which rabbits received four intramuscular injections of plasmid pWRG / c7d11 (H+L) (1 mg / injection, 4 mg total).
[0042]As shown in FIG. 1 below, these tests demonstrated that the lipid formulation of a plasmid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com