Method for determining baculovirus titer
A baculovirus and titer technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of insignificant cell lesions, subjective factors, and inability to distinguish between dead and alive viruses, and achieve good repeatability and accurate detection results Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Determination of Bac-HANA strain recombinant baculovirus titer
[0032] (1) Material
[0033] Sf9 cell is a commonly used insect cell, purchased from Invitrogen Company; AcMNPV-based recombinant baculovirus Bac-HANA (a recombinant baculovirus expressing avian influenza HA and NA genes, the construction method is shown in the application number CN200910104637.4 patent); gp64 monoclonal antibody is the product of ebioscience; goat anti-mouse fluorescent antibody is the product of Thermo; SF900Ⅱ serum-free medium is the product of Gibco; PBS (0.01M, pH7.2).
[0034] (2) Operation method
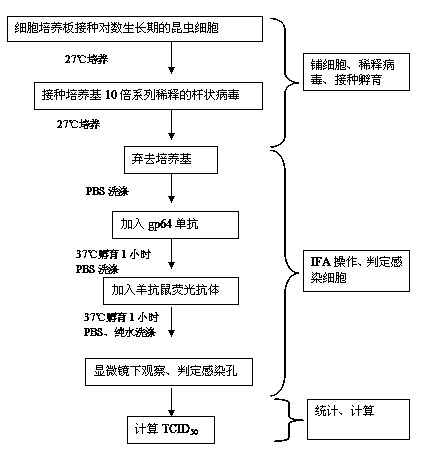
[0035] 1) Preparation of Sf9 cell plate: Sf9 cells were cultured in suspension in shake flasks with SF900II serum-free medium, the rotation speed of the shaker was 110 rpm, and the culture temperature was 27°C. Sf9 cells in the logarithmic growth phase and in suspension culture were diluted with SF900Ⅱ serum-free medium, and the diluted cell solution was diluted with 3×10 4 S...
Embodiment 2
[0044] Example 2 Determination of Bac-2ORF2 strain recombinant baculovirus titer
[0045] (1) Material
[0046] Sf9 cell is a commonly used insect cell, purchased from Invitrogen Company; AcMNPV-based recombinant baculovirus rBac-2ORF2 (a recombinant baculovirus expressing circovirus Cap protein, the construction method sees the application number CN201210270504.6 patent); gp64 monoclonal antibody is a product of ebioscience; cap protein monoclonal antibody is a product of American RTI; goat anti-mouse fluorescent antibody is a product of Thermo; SF900Ⅱ serum-free medium is a product of Gibco; PBS (0.01M, pH7.2) .
[0047] (2) Operation method
[0048] 1) Preparation of Sf9 cell plate: Sf9 cells were cultured in suspension in shake flasks with SF900II serum-free medium, the rotation speed of the shaker was 110 rpm, and the culture temperature was 27°C. Sf9 cells in the logarithmic growth phase and in suspension culture were diluted with SF900Ⅱ serum-free medium, and the dil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com