Human adiponectin ELISA kit
A kind of adiponectin and kit technology, applied in the field of medical immunology human adiponectin monoclonal antibody engineering, can solve the problems of high price, long operation time, and unfavorable large-scale detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
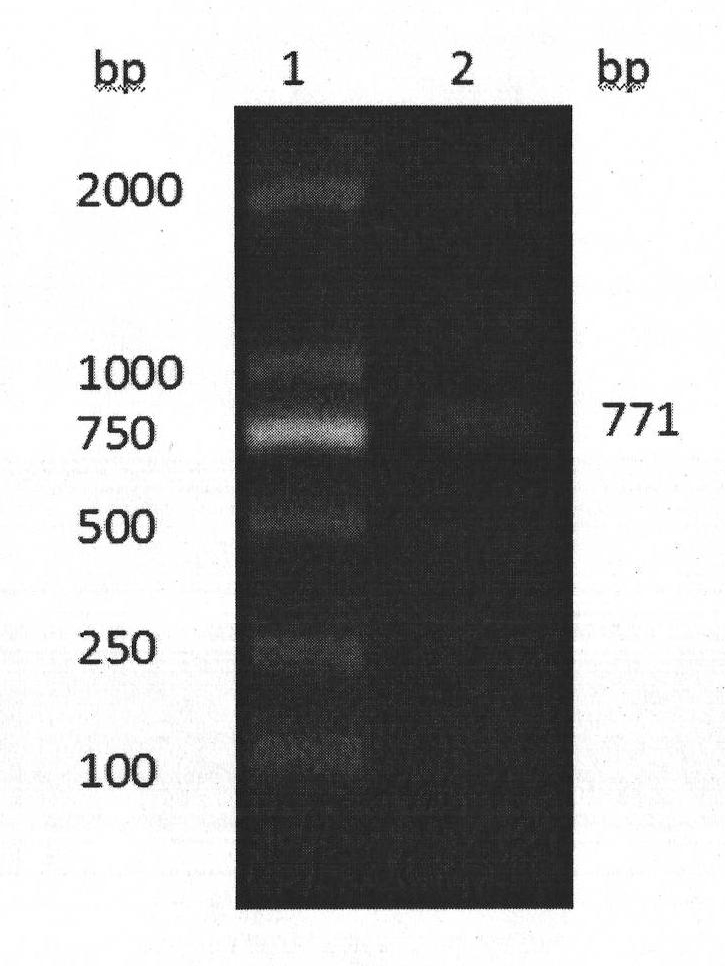
[0041] Example 1 Cloning Construction and Sequence Analysis of Human Adiponectin Gene
[0042] experiment material:
[0043]Horseradish peroxidase (HRP) is a product of Shanghai Sangong, and plasmid pMD 18-T, TaqDNA polymerase, Kpn I enzyme, EcoRI enzyme, T4 DNA ligase and DNAMarker are products of Dalian Bao Biological Company. Plasmid small mention medium extraction kit and DNA gel recovery kit are products of Omiga Company. The vectors pCDNA3.1(+) and Trizol are products of Invitrogen Company. M-MLV reverse transcriptase is a promega product. Mouse anti-human adiponectin monoclonal antibody was purchased from CST Company; Ni-NTA purification column Qiagen Company; human embryonic kidney cells (human embryonic kidney cells, HEK293) were purchased from American Type Culture Collection (ATCC); and DMEM high pond The culture medium and fetal bovine serum are products of Hycolon; the transfection reagent FuGENE HD is a product of Roche; dithiothreitol (DTT), DEPC water, G418,...
Embodiment 2
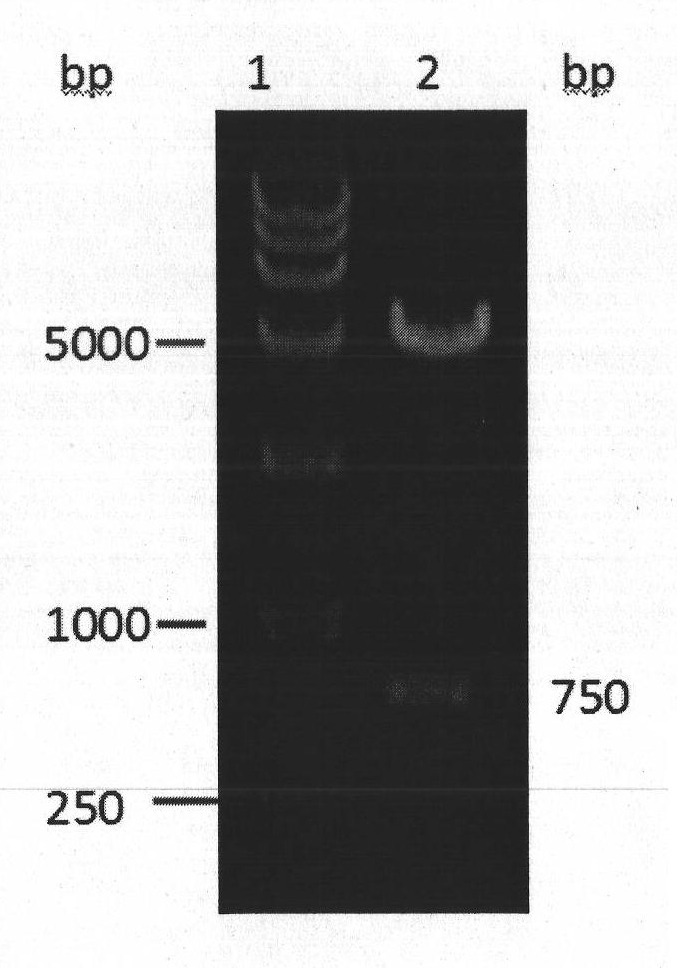
[0059] Embodiment 2 Preparation of recombinant human adiponectin
[0060] 1. Extraction of plasmid (pcDNA3.1-APN): Take a small amount of plasmid-containing glycerol bacterial solution stored at -80°C, draw on LB plate (100μg / ml ampicillin), activate overnight at 37°C, pick a single clone, and inoculate in 3ml LB medium (100 μg / ml ampicillin) was cultivated overnight at 37°C, and 1 mL of the culture solution was taken to inoculate fresh LB medium (100 μg / ml ampicillin) at a ratio of 1:50, and cultured at 37°C for 12 hours. The bacteria were collected by centrifugation, and the plasmid (pcDNA3.1-APN) was extracted using a plasmid extraction kit. The concentration was determined by UV spectrophotometer.
[0061] 2. Recombinant pcDNA3.1-APN stably transfected human embryonic kidney cells (human embryonic kidney cells, HEK293): HEK293 cells were cultured in a 35mm cell culture dish to 80% confluence (3-6×10 5 cells / 35mm Petri dish). According to the instructions of FuGENE HD Tr...
Embodiment 3
[0070] Embodiment 3 Preparation of mouse anti-human adiponectin monoclonal antibody
[0071] Taking XA-187-1 as an example, the preparation steps of mouse anti-human adiponectin monoclonal antibody polymer are as follows:
[0072] Step 1, animal immunization
[0073] Dissolve 150 μg of purified human recombinant adiponectin protein in 1.5 ml of normal saline, mix it with an equal amount of Freund’s complete adjuvant (purchased from Sigma) and fully emulsify it, inject BALB / c mice subcutaneously in multiple points on the back, and immunize 3 mice Eight-week-old female BALB / c mice were immunized for the second and third times at intervals of 4 weeks and 3 weeks, in which Freund's incomplete adjuvant was used for the second immunization, and no adjuvant was added for the third immunization. The immunization route was intraperitoneal injection. 10 days after the third immunization, the indirect ELISA method was used to detect the serum titer (enzyme-cleaved and purified protein co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com