ROS Kinase in Lung Cancer
a technology of ros kinase and lung cancer, which is applied in the direction of instruments, drug compositions, peptides, etc., can solve the problems of lung cancer and inability to respond completely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of ROS Kinase Activity in an NSCLC Cell Line by Global Phosphopeptide Profiling
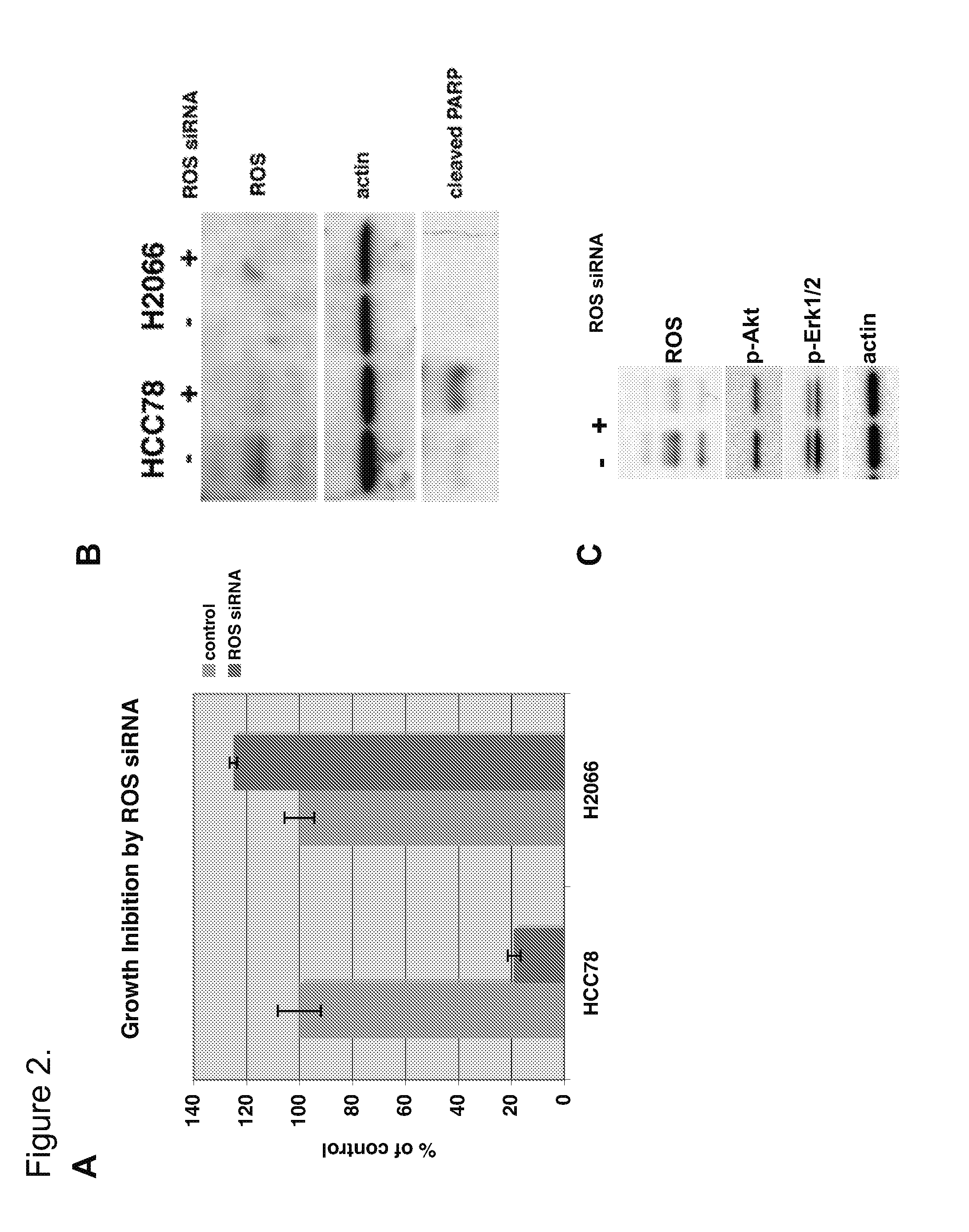
[0236]The global phosphorylation profile of kinase activation in several human NSCLC cell lines, including HCC78, were examined using the IAP technique for the isolation and mass spectrometric characterization of modified peptides from complex mixtures (see U.S. Pat. Nos. 7,300,753 and 7,198,896). The IAP technique was performed using a phosphotyrosine-specific antibody (commercially available from CELL SIGNALING TECHNOLOGY, INC., Danver, Mass., Cat. #9411) to isolate, and subsequently characterize, phosphotyrosine-containing peptides from extracts of the NSCLC cell lines.
[0237]Specifically, the IAP approach was employed to facilitate the identification of activated tyrosine kinases in the NSCLC cell lines, in order to identify novel drivers of this disease.
Cell Culture.
[0238]HCC78 cells were obtained from DSMZ (the German National Resource Centre for Biological Material), grown in RPMI-164...
example 2
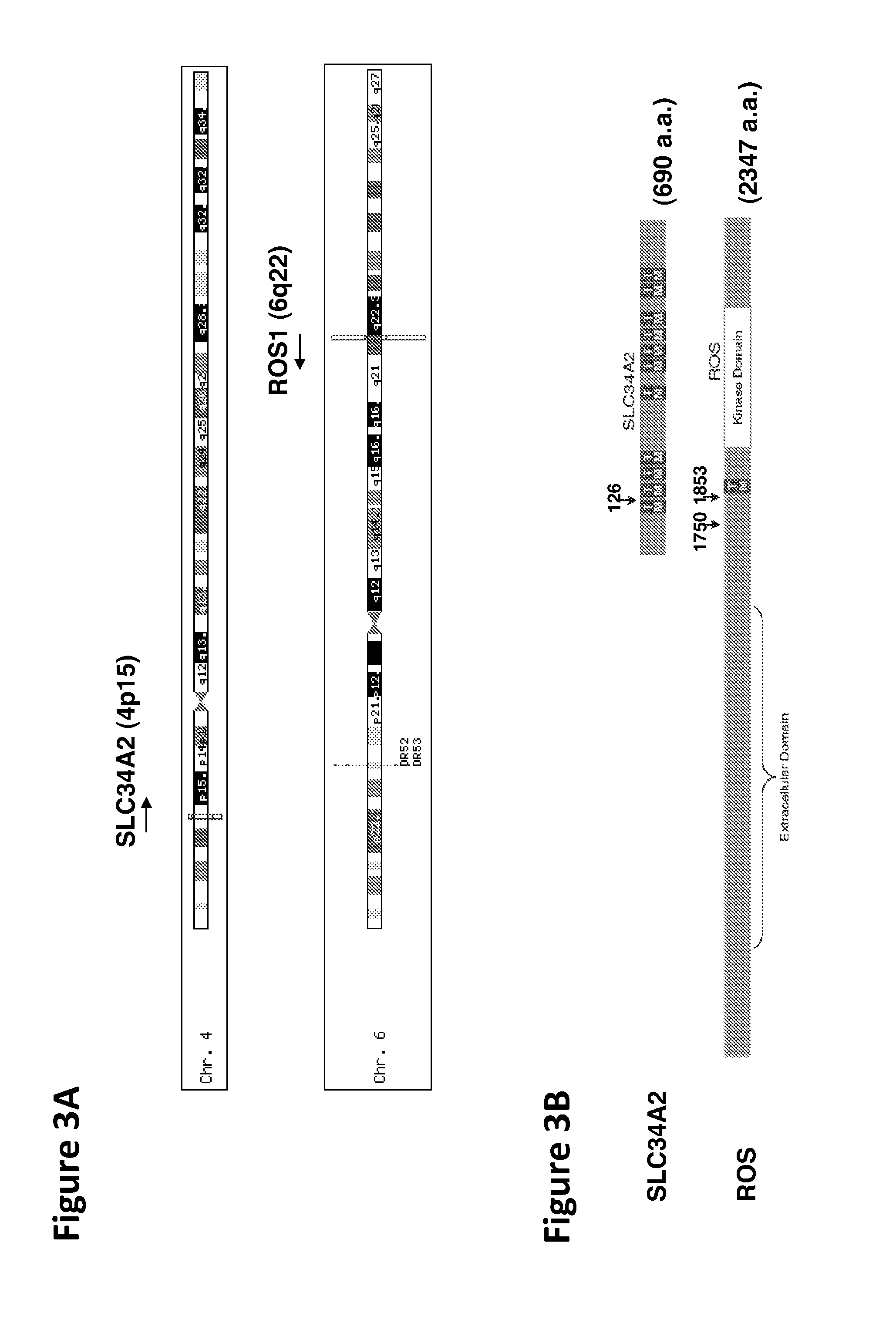
Detection of Mutant ROS Kinase Expression in a Human Cancer Sample Using Global Phosphopeptide Profiling
[0250]In order to further confirm the incidence of the ROS fusion mutation in human NSCLC, several human NSCLC tumors were examined, using the IAP technique of global phosphopeptide profiling described above (see Example 1), to identify ROS phosphopeptides in these tumors. Tumor samples (dissected tumors snap frozen and kept in liquid nitrogen) were obtained from a clinical collaborator in China (Second Xiangya Hospital, Central South University Changsha, Hunan, P.R. China).
[0251]Briefly, between about 300 milligrams-500 milligrams of tumor tissue were homogenized. For example, the tissue was homogenized and lysed in urea lysis buffer (20 mM HEPES pH 8.0, 9M urea, 1 mM sodium vanadate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate) at 1.25×108 cells / ml and sonicated. Sonicated lysates were cleared by centrifugation at 20,000×g, and proteins were reduced and alkylated as ...
example 3
Western Blot Analysis of ROS Kinase Expression in an NSCLC Cell Line
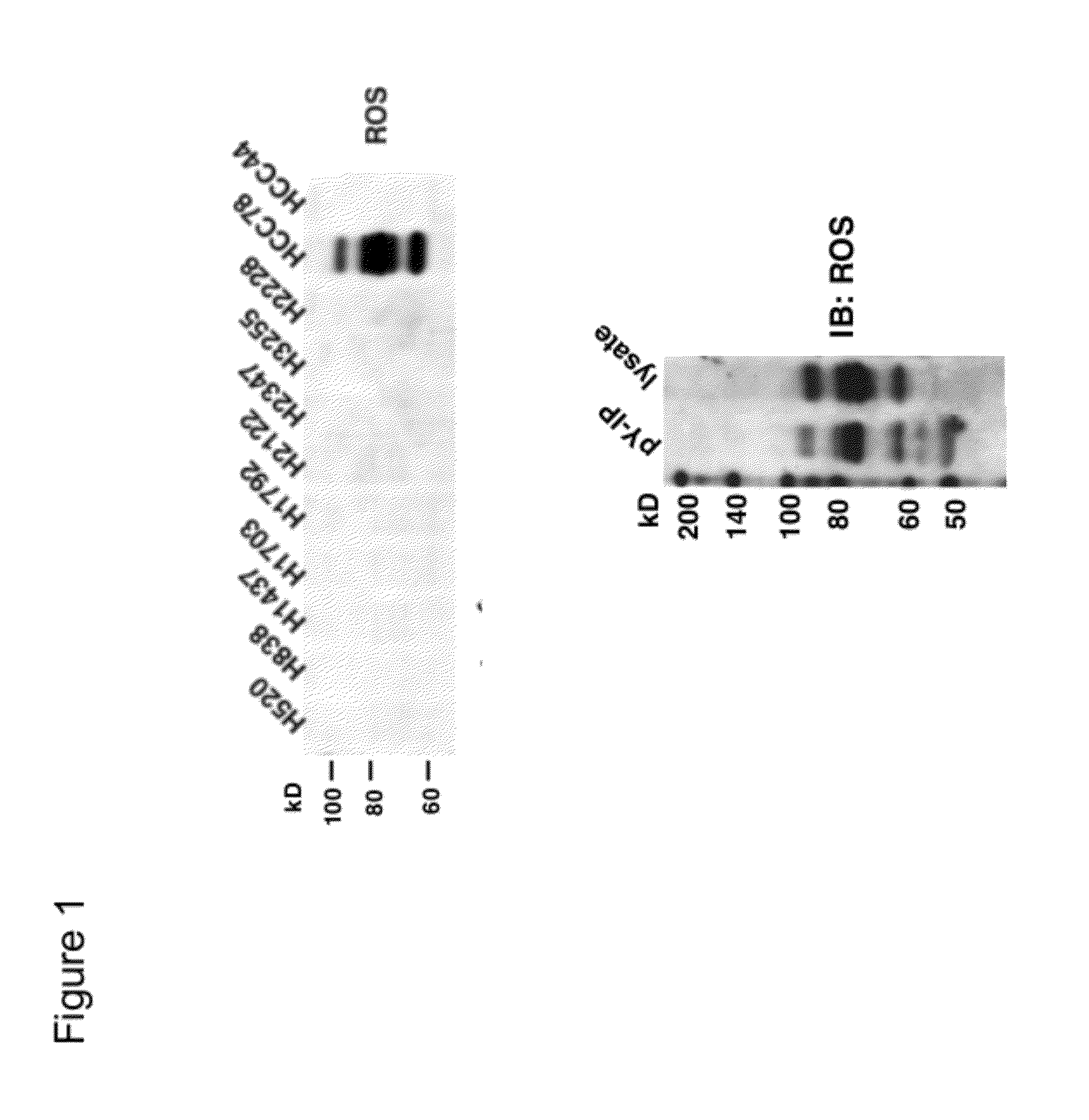
[0253]The observation that the HCC78 NSCLC cell line—but not the other NSCLC cell lines—expresses activated ROS kinase was confirmed by Western blot analysis of cell extracts using antibodies specific for ROS and other receptor tyrosine kinases (RTKs) and downstream kinases.
[0254]HCC78 cells were lysed in 1× cell lysis buffer (Cell Signaling Technology) supplemented with Protease Arrest™ (G Biosciences) and separated by electrophoresis. All antibodies and reagents for immunoblotting were from Cell Signaling Technology, Inc. (Danvers, Mass.). Western blotting was carried out as described in “Western Immunoblotting Protocol” (Cell Signaling Technology, Inc., 2005-2006 catalogue). Anti-ROS antibody was obtain from Santa Cruz Biotechnology, Inc.
[0255]FIG. 1 shows the western blot results. Only HCC78 express ROS protein among many different NSCLC cell lines. ROS protein in HCC78 has much smaller molecular weight than wil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com