Portable copper ion concentration detection method based on click chemistry
A portable detection and click chemistry technology, applied in the field of analytical chemistry, can solve the problems of inability to realize on-site detection, inconvenient to carry, and expensive, and achieve the effects of good activity and stability, easy control, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Synthesis of DNA compounds modified with alkynyl and sucrose invertase. References (Hermanson, G. T. Bioconjugate Techniques; Elsevier: London, 2008). Specific steps are as follows:
[0029] A. Mix 30 μM sulfhydryl-modified DNA with 60 μM TCEP in PBS buffer solution and incubate at room temperature for 1 hour;
[0030] B. Mix 11.30 mg / mL sucrose invertase and 1 mg Sulfo-SMCC in a buffer solution (0.1 M PBS, PH=7.3, 0.1 M NaCl) for 5 minutes, shake on a shaker at room temperature for 1 hour, and then centrifuge the mixture Separate and take the supernatant;
[0031] C. Mix the substances prepared in A and B, and react at room temperature for 48 hours.
[0032] 2. The preparation of magnetic beads modified with sucrose invertase and the detection of copper ions, the specific steps are as follows:
[0033](1) Dissolve the magnetic beads modified with streptavidin in a buffer solution consisting of 0.1M NaCl, 0.1M PBS with pH=7.3, and 0.05% Tween-20, add 0.3 μM DNA m...
Embodiment 2
[0038] The detection of copper ion concentration in human serum, the specific steps are as follows:
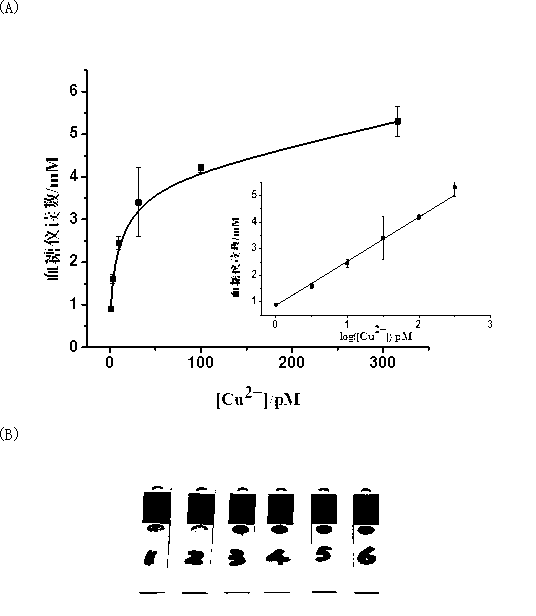
[0039] Add 0.6 mM sodium ascorbate to 0.3 μM mixture of the two modified DNAs and dilute 10 5 Double human serum sample, incubated at room temperature for (1.5±0.05) hours, then separated the mixture with a magnet, washed the magnetic beads with 50 μL buffer solution three times and added 10 μL buffer solution, then added 20 μL 1M sucrose solution, reacted at 40 °C for 15 minutes, and transferred Take 2.5 μL to measure with a blood glucose meter and record the data. According to the linear equation Y=0.86807+1.66028lgX of the copper ion concentration obtained in Example 1 and the blood glucose meter reading, the copper ion concentration in the four groups of human serum samples is calculated to be 19.23 pM, 16.74 pM, 16.74 pM, 16.74 pM, 16.74 pM.
Embodiment 3
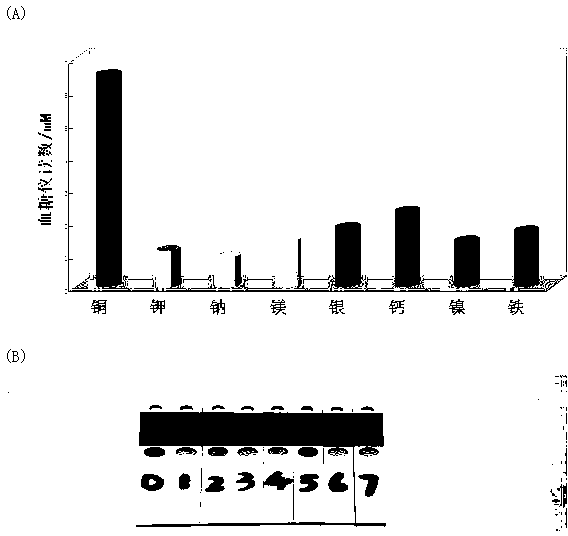
[0041] In order to detect the specificity of the present invention, the copper ions used in step 2 of Example 1 are replaced with other interfering substances, which are respectively potassium ions, sodium ions, magnesium ions, silver ions, calcium ions, nickel ions and iron ions, and copper ions The concentration of ions and other interfering species was 1 nM. Such as figure 2 (A), from left to right are the blood glucose meter signals shown in different interfering substances, from left to right are copper ions (6.5 mM), potassium ions (1.1 mM), sodium ions (0.9 mM), Magnesium ions (1.4 mM), silver ions (1.8 mM), calcium ions (2.3 mM), nickel ions (1.4 mM) and iron ions (1.7 mM), the corresponding color changes of the color windows of blood glucose test strips are as follows: figure 2 As shown in (B), from 0 to 7 are copper ions, potassium ions, 6.5 sodium ions, magnesium ions, silver ions, calcium ions, nickel ions and iron ions, except for 0 which is obviously green, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com